Characterizing altruistic motivation in potential volunteers for SARS-CoV-2 challenge trials
- PMID: 36322529
- PMCID: PMC9629635
- DOI: 10.1371/journal.pone.0275823
Characterizing altruistic motivation in potential volunteers for SARS-CoV-2 challenge trials
Abstract
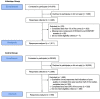
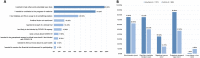
In human challenge trials (HCTs), volunteers are deliberately infected with an infectious agent. Such trials can be used to accelerate vaccine development and answer important scientific questions. Starting early in the COVID-19 pandemic, ethical concerns were raised about using HCTs to accelerate development and approval of a vaccine. Some of those concerns pertained to potential exploitation of and/or lack of truly informed consent from volunteers. Specific areas of concern arose around individuals who may be unusually risk-seeking or too economically vulnerable to refuse the payments these trials provide, as opposed to being motivated primarily by altruistic goals. This pre-registered study is the first large-scale survey to characterize people who, early in the pandemic, expressed interest and intention to volunteer to participate in COVID-19 HCTs. We found that individuals expressing interest in SARS-CoV-2 HCTs exhibit consistently altruistic motivations without any special indication of poor risk perception or economic vulnerability. In finding that, early in the pandemic, COVID-19 HCTs were able to attract volunteers whose values align with the nature of these trials, and who are not unusually vulnerable to exploitation, this study may allay some ethical concerns about the volunteers interested in participating in such trials.
Conflict of interest statement
The authors have read the journal’s policy and have the following competing interests: SMR and VS were employees of 1Day Sooner at the time of this study. JM is a current employee of 1Day Sooner. NE and AAM are on the Board of Advisors for 1Day Sooner. This does not alter our adherence to PLOS ONE policies on sharing data and materials. There are no patents, products in development or marketed products associated with this research to declare.
Figures
References
-
- World Health Organization. Key criteria for the ethical acceptability of COVID-19 human challenge studies [Internet]. World Health Organization; 2020. [cited 2022 Feb 17]. Report No.: WHO/2019-nCoV/Ethics_criteria/2020.1. Available from: https://apps.who.int/iris/handle/10665/331976
-
- Meiring JE, Giubilini A, Savulescu J, Pitzer VE, Pollard AJ. Generating the Evidence for Typhoid Vaccine Introduction: Considerations for Global Disease Burden Estimates and Vaccine Testing Through Human Challenge. Clin Infect Dis. 2019. Oct 15;69(Supplement_5):S402–7. doi: 10.1093/cid/ciz630 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

