σ-GeH and Germyl Cationic Pt(II) Complexes
- PMID: 36322561
- PMCID: PMC9949701
- DOI: 10.1021/acs.inorgchem.2c03186
σ-GeH and Germyl Cationic Pt(II) Complexes
Abstract
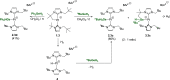
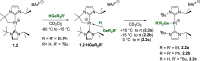
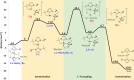
The low electron count Pt(II) complexes [Pt(NHC')(NHC)][BArF] (where NHC is a N-heterocyclic carbene ligand and NHC' its metalated form) react with tertiary hydrogermanes HGeR3 at room temperature to generate the 14-electron platinum(II) germyl derivatives [Pt(GeR3)(NHC)2][BArF]. Low-temperature NMR studies allowed us to detect and characterize spectroscopically some of the σ-GeH intermediates [Pt(η2-HGeR3)(NHC')(NHC)][BArF] that evolve into the platinum-germyl species. One of these compounds has been characterized by X-ray diffraction studies, and the interaction of the H-Ge bond with the platinum center has been analyzed in detail by computational methods, which suggest that the main contribution is the donation of the H-Ge to a σ*(Pt-C) orbital, but backdonation from the platinum to the σ*(Ge-H) orbital is significant. Primary and secondary hydrogermanes also produce the corresponding platinum-germyl complexes, a result that contrasts with the reactivity observed with primary silanes, in which carbon-silicon bond-forming reactions have been reported. According to density functional theory calculations, the formation of Pt-Ge/C-H bonds is both kinetically and thermodynamically preferred over the competitive reaction pathway leading to Pt-H/C-Ge bonds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures










Similar articles
-
σ-Silane Platinum(II) Complexes as Intermediates in C-Si Bond-Coupling Processes.Chemistry. 2019 Aug 27;25(48):11346-11355. doi: 10.1002/chem.201902226. Epub 2019 Aug 5. Chemistry. 2019. PMID: 31246316
-
Reactivity of coordinatively unsaturated bis(N-heterocyclic carbene) Pt(II) complexes toward H(2). Crystal structure of a 14-electron Pt(II) hydride complex.Inorg Chem. 2014 Apr 21;53(8):4257-68. doi: 10.1021/ic500705t. Epub 2014 Apr 9. Inorg Chem. 2014. PMID: 24716606
-
Cationic Platinum(II) σ-SiH Complexes in Carbon Dioxide Hydrosilation.Chemistry. 2016 Nov 14;22(47):16791-16795. doi: 10.1002/chem.201603524. Epub 2016 Oct 11. Chemistry. 2016. PMID: 27662609
-
Dehydrogenation of saturated CC and BN bonds at cationic N-heterocyclic carbene stabilized M(III) centers (M = Rh, Ir).J Am Chem Soc. 2010 Aug 4;132(30):10578-91. doi: 10.1021/ja1043787. J Am Chem Soc. 2010. PMID: 20662531
-
Isolation of a Cationic Platinum(II) σ-Silane Complex.Angew Chem Int Ed Engl. 2018 Mar 12;57(12):3217-3221. doi: 10.1002/anie.201712791. Epub 2018 Feb 22. Angew Chem Int Ed Engl. 2018. PMID: 29384245
References
-
- Whited M. T.; Taylor B. L. H. Metal/organosilicon complexes: structure, reactivity, and considerations for catalysis. Comments Inorg. Chem. 2020, 40, 217–276. 10.1080/02603594.2020.1737026. - DOI
- Corey J. Y. Reactions of hydrosilanes with transition metal complexes. Chem. Rev. 2016, 116, 11291–11435. 10.1021/acs.chemrev.5b00559. - DOI - PubMed
- Riddlestone I. M.; Abdalla J. A. B.; Aldridge S. Coordination and activation of E–H Bonds (E = B, Al, Ga) at transition metal centers. Adv. Organomet. Chem. 2015, 63, 1–38.
- Corey J. Y. Reactions of hydrosilanes with transition metal complexes and characterization of the products. Chem. Rev. 2011, 111, 863–1071. 10.1021/cr900359c. - DOI - PubMed
- Pandey K. K. Transition metal σ-borane complexes. Coord. Chem. Rev. 2009, 253, 37–55. 10.1016/j.ccr.2007.11.026. - DOI
- Alcaraz G.; Grellier M.; Sabo-Etienne S. Bis σ-bond dihydrogen and borane ruthenium complexes: bonding nature, catalytic applications, and reversible hydrogen release. Acc. Chem. Res. 2009, 42, 1640–1649. 10.1021/ar900091a. - DOI - PubMed
- Nikonov G. I. Recent advances in nonclassical interligand Si···H interactions. Adv. Organomet. Chem. 2005, 53, 217–309.
- Kubas G. J. Heterolytic splitting of H–H, Si–H, and other σ bonds on electrophilic metal centers. Adv. Inorg. Chem. 2004, 56, 127–177.
-
- Handzlik J.; Kochel A.; Szymańska-Buzar T. H–Ge bond activation by tungsten carbonyls: An experimental and theoretical study. Polyhedron 2012, 31, 622–637. 10.1016/j.poly.2011.10.040. - DOI
- Zyder M.; Szymańska-Buzar T. Activation of the Ge–H bond of Et3GeH in photochemical reaction with molybdenum(0) carbonyl complexes and hydrogermylation of norbornadiene. J. Organomet. Chem. 2009, 694, 2110–2113. 10.1016/j.jorganchem.2009.02.013. - DOI
- Sabo-Etienne S.; Hernandez M.; Chung G.; Chaudret B.; Castel A. Substitution reactions of coordinated dihydrogen by weakly coordinating ligands: preparation of RuH2(N2)2(PCy3)2 and RuH2(η2-H2)(HEPh3)(PCy3)2 (E = Si, Ge). New J. Chem. 1994, 18, 175–177.
- Lichtenberger D. L.; Rai-Chaudhuri A. Electronic structure factors of Ge–H bond activation by transition metals. Photoelectron spectra of [Mn(η5-C5H5)(CO)2(HGePh3)], [Mn(η5-C5H4Me)(CO)2(HGePh3)], and [Mn(η5-C5Me5)(CO)2(HGePh3)]. J. Chem. Soc., Dalton Trans. 1990, 2161–2166. 10.1039/DT9900002161. - DOI
- Garré F.; Colomer E.; Corriu R. J. P.; Vioux A. Stereochemistry of the insertion of manganese into Si–H and Ge–H Bonds. Complexes containing a two-electron, three-center Mn···H···Si (or Ge) interaction. Organometallics 1984, 3, 1272–1278. 10.1021/om00086a022. - DOI
-
- Smart K. A.; Mothes-Martin E.; Vendier L.; Perutz R. N.; Grellier M.; Sabo-Etienne S. A ruthenium dihydrogen germylene complex and the catalytic synthesis of digermoxane. Organometallics 2015, 34, 4158–4163. 10.1021/acs.organomet.5b00570. - DOI
- Vincent J. L.; Luo S.; Scott B. L.; Butcher R.; Unkefer C. J.; Burns C. J.; Kubas G. J.; Lledós A.; Maseras F.; Tomàs J. Oxidative addition of germanes and silanes, EH4-nPhn (E = Si, Ge; n = 0-3), to Mo(CO)(diphosphine)2. The first structurally characterized germane σ complex. Organometallics 2003, 22, 5307–5323. 10.1021/om030569j. - DOI
-
For “agostic” (mono- and di-nuclear) complexes see:
- Takaya J.; Iwasawa N. Synthesis, structure, and reactivity of a mononuclear η2-(Ge–H)-palladium(0) complex bearing a PGeP-pincer-type germyl ligand: reactivity differences between silicon and germanium. Eur. J. Inorg. Chem. 2018, 2018, 5012–5018. 10.1002/ejic.201801257. - DOI
- Mobarok M. H.; McDonald R.; Ferguson M. J.; Cowie M. Germyl- and germylene-bridged complexes of Rh/Ir and subsequent chemistry of a bridging germylene group. Inorg. Chem. 2012, 51, 4020–4034. 10.1021/ic2021269. - DOI - PubMed
- Zyder M.; Kochel A.; Handzlik J.; Szymańska-Buzar T. Photochemical reaction of Mo(CO)6 with Et2GeH2: NMR and DFT studies of reaction products; crystal structure of a novel complex [{Mo(μ-η2-H-GeEt2)(CO)4}2]. Organometallics 2009, 28, 5857–5865. 10.1021/om900299k. - DOI
- Tanabe M.; Ishikawa N.; Osakada K. Preparation and structure of a new dipalladium complex with bridging diphenylgermyl ligands. Diverse reactivities of Pd(PCy3)2 and Pt(PCy3)2 toward Ph2GeH2. Organometallics 2006, 25, 796–798. 10.1021/om050981u. - DOI
- Braddock-Wilking J.; Corey J. Y.; White C.; Xu H.; Rath N. P. Reaction of diphenylgermane with (Ph3P)2Pt(η2-C2H4): generation of mono- and dinuclear complexes containing Pt–Ge bonds. X-ray crystal structure determination of [(Ph3P)Pt(μ-η2-H-GePh2)]2. Organometallics 2005, 24, 4113–4115. 10.1021/om050392o. - DOI
-
- Ríos P.; Martín-de la Calle R.; Vidossich P.; Fernández-de-Córdova F. J.; Lledós A.; Conejero S. Reversible carbon–boron bond formation at platinum centers through σ-BH complexes. Chem. Sci. 2021, 12, 1647–1655. 10.1039/D0SC05522K. - DOI - PMC - PubMed
- Ríos P.; Fouilloux H.; Díez J.; Vidossich P.; Lledós A.; Conejero S. σ-Silane platinum(II) complexes as intermediates in C–Si bond- coupling processes. Chem. - Eur. J. 2019, 25, 11346–11355. 10.1002/chem.201902226. - DOI - PubMed
- Ríos P.; Fouilloux H.; Vidossich P.; Díez J.; Lledós A.; Conejero S. Isolation of a cationic platinum(II) σ-silane complex. Angew. Chem., Int. Ed. 2018, 57, 3217–3221. 10.1002/anie.201712791. - DOI - PubMed
- Ríos P.; Díez J.; López-Serrano J.; Rodríguez A.; Conejero S. Cationic platinum(II) σ-SiH complexes in carbon dioxide hydrosilation. Chem. - Eur. J. 2016, 22, 16791–16795. 10.1002/chem.201603524. - DOI - PubMed
- Rivada-Wheelaghan O.; Roselló-Merino M.; Ortuño M. A.; Vidossich P.; Gutiérrez-Puebla E.; Lledós A.; Conejero S. Reactivity of coordinatively unsaturated bis(N-heterocyclic carbene) Pt(II) complexes toward H2. Crystal structure of a 14-electron Pt(II) hydride complex. Inorg. Chem. 2014, 53, 4257–4268. 10.1021/ic500705t. - DOI - PubMed
- Ríos P.; Fernández-de-Córdova F. J.; Borge J.; Curado N.; Lledós A.; Conejero S. Ligand effects in carbon-boron coupling processes mediated by σ-BH platinum complexes. Eur. J. Inorg. Chem. 2021, 2021, 3528–3539. 10.1002/ejic.202100428. - DOI
-
-
See for example:
- Selmani A.; Schoetz M. D.; Queen A. E.; Schoenebeck F. Modularity in the Csp3 space–alkyl germanes as orthogonal molecular handles for chemoselective diversification. ACS Catal. 2022, 12, 4833–4839. 10.1021/acscatal.2c00852. - DOI
- Dahiya A.; Schoenebeck F. Direct C–H dehydrogenative germylation of terminal alkynes with hydrogermanes. Org. Lett. 2022, 24, 2728–2732. 10.1021/acs.orglett.2c00840. - DOI - PubMed
- Xu Q.-H.; Wei L.-P.; Xiao B. Alkyl-GeMe3: neutral metalloid radical precursors upon visible light photocatalysis. Angew. Chem., Int. Ed. 2022, 61, e202115592. - PubMed
- Luo Y.; Xu B.; Lv L.; Li Z. Copper-catalyzed three-component germyl peroxidation of alkenes. Org. Lett. 2022, 24, 2425–2430. 10.1021/acs.orglett.2c00698. - DOI - PubMed
- Su P.-F.; Wang K.; Peng X.; Pang X.; Guo P.; Shu X.-Z. Nickel-catalyzed reductive C–Ge coupling of aryl/alkenyl electrophiles with chlorogermanes. Angew. Chem., Int. Ed. 2021, 60, 26571–2657. 10.1002/anie.202112876. - DOI - PubMed
- Kojima K.; Uchida S.; Kinoshita H.; Miura K. Synthesis of polysubstituted germoles and benzogermoles using a substoichiometric amount of diisobutylaluminum hydride. Org. Lett. 2021, 23, 4598–4602. 10.1021/acs.orglett.1c01314. - DOI - PubMed
- Fricke C.; Schoenebeck F. Organogermanes as orthogonal coupling partners in synthesis and catalysis. Acc. Chem. Res. 2020, 53, 2715–2725. 10.1021/acs.accounts.0c00527. - DOI - PubMed
- Sherborne G. J.; Gevondian A. G.; Funes-Ardoiz I.; Dahiya A.; Fricke C.; Schoenebeck F. Modular and selective arylation of aryl germanes (C–GeEt3) over C–Bpin, C–SiR3 and halogens enabled by light-activated gold catalysis. Angew. Chem., Int. Ed. 2020, 59, 15543–15548. 10.1002/anie.202005066. - DOI - PMC - PubMed
- Liang H.; Ji Y.-X.; Wang R.-H.; Zhang Z.-H.; Zhang B. Visible-light-initiated manganese-catalyzed E-selective hydrosilylation and hydrogermylation of alkynes. Org. Lett. 2019, 21, 2750–2754. 10.1021/acs.orglett.9b00701. - DOI - PubMed
- Xue W.; Mao W.; Zhang L.; Oestreich M. Mechanistic dichotomy of magnesium- and zinc-based germanium nucleophiles in the C(sp3)–Ge cross-coupling with alkyl electrophiles. Angew. Chem., Int. Ed. 2019, 58, 6440–6443. 10.1002/anie.201901860. - DOI - PubMed
- Keess S.; Oestreich M. Access to fully alkylated germanes by B(C6F5)3-catalyzed transfer hydrogermylation of alkenes. Org. Lett. 2017, 19, 1898–1901. 10.1021/acs.orglett.7b00672. - DOI - PubMed
-
LinkOut - more resources
Full Text Sources

