Lipids mediate supramolecular outer membrane protein assembly in bacteria
- PMID: 36322653
- PMCID: PMC9629720
- DOI: 10.1126/sciadv.adc9566
Lipids mediate supramolecular outer membrane protein assembly in bacteria
Abstract
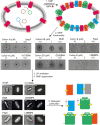
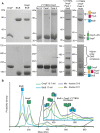
β Barrel outer membrane proteins (OMPs) cluster into supramolecular assemblies that give function to the outer membrane (OM) of Gram-negative bacteria. How such assemblies form is unknown. Here, through photoactivatable cross-linking into the Escherichia coli OM, coupled with simulations, and biochemical and biophysical analysis, we uncover the basis for OMP clustering in vivo. OMPs are typically surrounded by an annular shell of asymmetric lipids that mediate higher-order complexes with neighboring OMPs. OMP assemblies center on the abundant porins OmpF and OmpC, against which low-abundance monomeric β barrels, such as TonB-dependent transporters, are packed. Our study reveals OMP-lipid-OMP complexes to be the basic unit of supramolecular OMP assembly that, by extending across the entire cell surface, couples the requisite multifunctionality of the OM to its stability and impermeability.
Figures



References
-
- Murray C. J., Ikuta K. S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., Johnson S. C., Browne A. J., Chipeta M. G., Fell F., Hackett S., Haines-Woodhouse G., Kashef Hamadani B. H., Kumaran E. A. P., McManigal B., Agarwal R., Akech S., Albertson S., Amuasi J., Andrews J., Aravkin A., Ashley E., Bailey F., Baker S., Basnyat B., Bekker A., Bender R., Bethou A., Bielicki J., Boonkasidecha S., Bukosia J., Carvalheiro C., Castañeda-Orjuela C., Chansamouth V., Chaurasia S., Chiurchiù S., Chowdhury F., Cook A. J., Cooper B., Cressey T. R., Criollo-Mora E., Cunningham M., Darboe S., Day N. P. J., de Luca M., Dokova K., Dramowski A., Dunachie S. J., Eckmanns T., Eibach D., Emami A., Feasey N., Fisher-Pearson N., Forrest K., Garrett D., Gastmeier P., Giref A. Z., Greer R. C., Gupta V., Haller S., Haselbeck A., Hay S. I., Holm M., Hopkins S., Iregbu K. C., Jacobs J., Jarovsky D., Javanmardi F., Khorana M., Kissoon N., Kobeissi E., Kostyanev T., Krapp F., Krumkamp R., Kumar A., Kyu H. H., Lim C., Limmathurotsakul D., Loftus M. J., Lunn M., Ma J., Mturi N., Munera-Huertas T., Musicha P., Mussi-Pinhata M. M., Nakamura T., Nanavati R., Nangia S., Newton P., Ngoun C., Novotney A., Nwakanma D., Obiero C. W., Olivas-Martinez A., Olliaro P., Ooko E., Ortiz-Brizuela E., Peleg A. Y., Perrone C., Plakkal N., Ponce-de-Leon A., Raad M., Ramdin T., Riddell A., Roberts T., Robotham J. V., Roca A., Rudd K. E., Russell N., Schnall J., Scott J. A. G., Shivamallappa M., Sifuentes-Osornio J., Steenkeste N., Stewardson A. J., Stoeva T., Tasak N., Thaiprakong A., Thwaites G., Turner C., Turner P., van Doorn H. R., Velaphi S., Vongpradith A., Vu H., Walsh T., Waner S., Wangrangsimakul T., Wozniak T., Zheng P., Sartorius B., Lopez A. D., Stergachis A., Moore C., Dolecek C., Naghavi M., Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 399, 629–655 (2022). - PMC - PubMed
-
- ECDC/EMEA Joint Working Group, The Bacterial Challenge: Time to React: A Call to Narrow the Gap Between Multidrug-Resistant Bacteria in the EU and the Development of New Antibacterial Agents (EUR-OP, 2009).
-
- Wimley W. C., The versatile beta-barrel membrane protein. Curr. Opin. Struct. Biol. 13, 404–411 (2003). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

