IER3IP1 is critical for maintaining glucose homeostasis through regulating the endoplasmic reticulum function and survival of β cells
- PMID: 36322741
- PMCID: PMC9659391
- DOI: 10.1073/pnas.2204443119
IER3IP1 is critical for maintaining glucose homeostasis through regulating the endoplasmic reticulum function and survival of β cells
Abstract
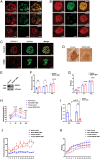
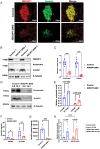
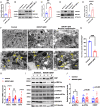
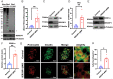
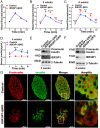
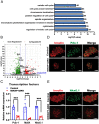
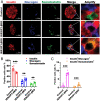
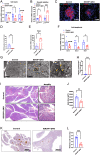
Recessive mutations in IER3IP1 (immediate early response 3 interacting protein 1) cause a syndrome of microcephaly, epilepsy, and permanent neonatal diabetes (MEDS). IER3IP1 encodes an endoplasmic reticulum (ER) membrane protein, which is crucial for brain development; however, the role of IER3IP1 in β cells remains unknown. We have generated two mouse models with either constitutive or inducible IER3IP1 deletion in β cells, named IER3IP1-βKO and IER3IP1-iβKO, respectively. We found that IER3IP1-βKO causes severe early-onset, insulin-deficient diabetes. Functional studies revealed a markedly dilated β-cell ER along with increased proinsulin misfolding and elevated expression of the ER chaperones, including PDI, ERO1, BiP, and P58IPK. Islet transcriptome analysis confirmed by qRT-PCR revealed decreased expression of genes associated with β-cell maturation, cell cycle, and antiapoptotic genes, accompanied by increased expression of antiproliferation genes. Indeed, multiple independent approaches further demonstrated that IER3IP1-βKO impaired β-cell maturation and proliferation, along with increased condensation of β-cell nuclear chromatin. Inducible β-cell IER3IP1 deletion in adult (8-wk-old) mice induced a similar diabetic phenotype, suggesting that IER3IP1 is also critical for function and survival even after β-cell early development. Importantly, IER3IP1 was decreased in β cells of patients with type 2 diabetes (T2D), suggesting an association of IER3IP1 deficiency with β-cell dysfunction in the more-common form of diabetes. These data not only uncover a critical role of IER3IP1 in β cells but also provide insight into molecular basis of diabetes caused by IER3IP1 mutations.
Keywords: ER stress; IER3IP1; diabetes; β cell proliferation; β cell survival.
Conflict of interest statement
The authors declare no competing interest.
Figures
References
-
- De Franco E., From biology to genes and back again: Gene discovery for monogenic forms of beta-cell dysfunction in diabetes. J. Mol. Biol. 432, 1535–1550 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

