Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy
- PMID: 36322757
- PMCID: PMC9659366
- DOI: 10.1073/pnas.2213162119
Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy
Abstract
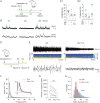
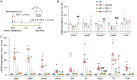
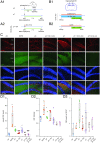
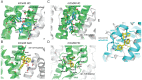
Temporal lobe epilepsy (TLE) is one of the most common types of epilepsy, yet approximately one-third of patients are refractory to current anticonvulsive drugs, which target neurons and synapses. Astrocytic and microglial dysfunction is commonly found in epileptic foci and has been shown to contribute to neuroinflammation and hyperexcitability in chronic epilepsy. Accumulating evidence points to a key role for glial hemichannels in epilepsy, but inhibiting both connexin (Cx) gap junctions and hemichannels can lead to undesirable side effects because the former coordinate physiological functions of cell assemblies. It would be a great benefit to use an orally available small molecule to block hemichannels to alleviate epileptic symptoms. Here, we explored the effect of D4, a newly developed compound that inhibits the Cx hemichannels but not Cx gap junctions using the pilocarpine mouse model of TLE. In vitro application of D4 caused a near-complete reduction in the pilocarpine-induced cell membrane permeability associated with increased Cx hemichannel activity. Moreover, preadministration of D4 in vivo effectively reduced neuroinflammation and altered synaptic inhibition, which then enhanced the animal survival rate. Posttreatment with a single dose of D4 in vivo has prolonged effects on suppressing the activation of astrocytes and microglia and rescued the changes in neuroinflammatory and synaptic gene expression induced by pilocarpine. Collectively, these results indicate that targeting Cx hemichannels by D4 is an effective and promising strategy for treating epilepsy in which neuroinflammation plays a critical role.
Keywords: antiepileptic drug; connexon; excitability; seizure.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Targeting connexin hemichannels to treat temporal lobe epilepsy.Nat Rev Neurol. 2023 Jan;19(1):1. doi: 10.1038/s41582-022-00752-4. Nat Rev Neurol. 2023. PMID: 36418496 No abstract available.
References
-
- García-Rodríguez C., Bravo-Tobar I. D., Duarte Y., Barrio L. C., Sáez J. C., Contribution of non-selective membrane channels and receptors in epilepsy. Pharmacol. Ther. 231, 107980 (2022). - PubMed
-
- Seifert G., Steinhäuser C., Neuron-astrocyte signaling and epilepsy. Exp. Neurol. 244, 4–10 (2013). - PubMed
-
- Bordey A., Lyons S. A., Hablitz J. J., Sontheimer H., Electrophysiological characteristics of reactive astrocytes in experimental cortical dysplasia. J. Neurophysiol. 85, 1719–1731 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

