Coupling-dependent metabolic ultradian rhythms in confluent cells
- PMID: 36322771
- PMCID: PMC9659342
- DOI: 10.1073/pnas.2211142119
Coupling-dependent metabolic ultradian rhythms in confluent cells
Abstract
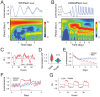
Ultradian rhythms in metabolism and physiology have been described previously in mammals. However, the underlying mechanisms for these rhythms are still elusive. Here, we report the discovery of temperature-sensitive ultradian rhythms in mammalian fibroblasts that are independent of both the cell cycle and the circadian clock. The period in each culture is stable over time but varies in different cultures (ranging from 3 to 24 h). We show that transient, single-cell metabolic pulses are synchronized into stable ultradian rhythms across contacting cells in culture by gap junction-mediated coupling. Coordinated rhythms are also apparent for other metabolic and physiological measures, including plasma membrane potential (Δψp), intracellular glutamine, α-ketoglutarate, intracellular adenosine triphosphate (ATP), cytosolic pH, and intracellular calcium. Moreover, these ultradian rhythms require extracellular glutamine, several different ion channels, and the suppression of mitochondrial ATP synthase by α-ketoglutarate, which provides a key feedback mechanism. We hypothesize that cellular coupling and metabolic feedback can be used by cells to balance energy demands for survival.
Keywords: cellular metabolism; gap junctions; ion channels; membrane potential; ultradian rhythms.
Conflict of interest statement
Competing interest statement: Dr. Hogenesch and Dr. Takahashi are co-authors on a consensus review article with 18 co-authors on circadian medicine published in 2019 in Cell Metabolism (PMID: 31390550). The authors declare no competing interest.
Figures



References
-
- Goldbeter A., Biological rhythms: Clocks for all times. Curr. Biol. 18, R751–R753 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

