One-Step Regio- and Stereoselective Electrochemical Synthesis of Orexin Receptor Antagonist Oxidative Metabolites
- PMID: 36322780
- PMCID: PMC10512451
- DOI: 10.1021/acs.joc.2c01311
One-Step Regio- and Stereoselective Electrochemical Synthesis of Orexin Receptor Antagonist Oxidative Metabolites
Abstract
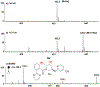
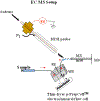
Synthesis of drug metabolites, which often have complex structures, is an integral step in the evaluation of drug candidate metabolism, pharmacokinetic (PK) properties, and safety profiles. Frequently, such synthetic endeavors entail arduous, multiple-step de novo synthetic routes. Herein, we present the one-step Shono-type electrochemical synthesis of milligrams of chiral α-hydroxyl amide metabolites of two orexin receptor antagonists, MK-8133 and MK-6096, as revealed by a small-scale (pico- to nano-mole level) reaction screening using a lab-built online electrochemistry (EC)/mass spectrometry (MS) (EC/MS) platform. The electrochemical oxidation of MK-8133 and MK-6096 was conducted in aqueous media and found to produce the corresponding α-piperidinols with exclusive regio- and stereoselectivity, as confirmed by high-resolution nuclear magnetic resonance (NMR) characterization of products. Based on density functional theory (DFT) calculations, the exceptional regio- and stereoselectivity for this electrochemical oxidation are governed by more favorable energetics of the transition state, leading to the preferred secondary carbon radical α to the amide group and subsequent steric hindrance associated with the U-shaped conformation of the cation derived from the secondary α-carbon radical, respectively.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
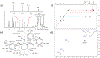


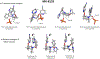


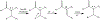
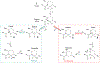
References
-
- Benca RM, Diagnosis and Treatment of Chronic Insomnia: A Review. Psych. Serv 2005, 56 (3), 332–343. - PubMed
-
- Compernolle F; Saleh MA; Van den Branden S; Toppet S; Hoornaert G, Regioselective oxidation of piperidine-3 derivatives: a synthetic route to 2,5-substituted piperidines. J. Org. Chem 1991, 56 (7), 2386–2390.
-
- Pelkonen O; Turpeinen M; Uusitalo J; Rautio A; Raunio H, Prediction of drug metabolism and interactions on the basis of in vitro investigations. Basic Clin. Pharmacol. Toxicol 2005, 96 (3), 167–175. - PubMed
-
- Plant N, Strategies for using in vitro screens in drug metabolism. Drug Discov. Today 2004, 9 (7), 328–336. - PubMed
-
- Brandon EFA; Raap CD; Meijerman I; Beijnen JH; Schellens JHM, An update on in vitro test methods in human hepatic drug biotransformation research: pros and cons. Toxicol. Appl. Pharmacol 2003, 189 (3), 233–246. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

