Sustained hematologic remission after discontinuation of sutimlimab treatment in patients with cold agglutinin disease
- PMID: 36322823
- PMCID: PMC10189382
- DOI: 10.1182/bloodadvances.2022008574
Sustained hematologic remission after discontinuation of sutimlimab treatment in patients with cold agglutinin disease
Conflict of interest statement
Figures
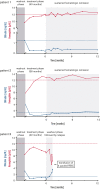
References
-
- Berentsen S, Barcellini W, D'Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136(4):480–488. - PubMed
-
- Jäger U, Barcellini W, Broome CM, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648. - PubMed
-
- Shi J, Rose EL, Singh A, et al. TNT003, an inhibitor of the serine protease C1s, prevents complement activation induced by cold agglutinins. Blood. 2014;123(26):4015–4022. - PubMed

