Covid-19 Vaccine Protection among Children and Adolescents in Qatar
- PMID: 36322837
- PMCID: PMC9644642
- DOI: 10.1056/NEJMoa2210058
Covid-19 Vaccine Protection among Children and Adolescents in Qatar
Abstract
Background: The BNT162b2 vaccine against coronavirus disease 2019 (Covid-19) has been authorized for use in children 5 to 11 years of age and adolescents 12 to 17 years of age but in different antigen doses.
Methods: We assessed the real-world effectiveness of the BNT162b2 vaccine against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among children and adolescents in Qatar. To compare the incidence of SARS-CoV-2 infection in the national cohort of vaccinated participants with the incidence in the national cohort of unvaccinated participants, we conducted three matched, retrospective, target-trial, cohort studies - one assessing data obtained from children 5 to 11 years of age after the B.1.1.529 (omicron) variant became prevalent and two assessing data from adolescents 12 to 17 years of age before the emergence of the omicron variant (pre-omicron study) and after the omicron variant became prevalent. Associations were estimated with the use of Cox proportional-hazards regression models.
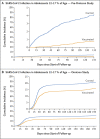
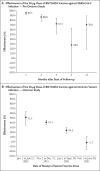
Results: Among children, the overall effectiveness of the 10-μg primary vaccine series against infection with the omicron variant was 25.7% (95% confidence interval [CI], 10.0 to 38.6). Effectiveness was highest (49.6%; 95% CI, 28.5 to 64.5) right after receipt of the second dose but waned rapidly thereafter and was negligible after 3 months. Effectiveness was 46.3% (95% CI, 21.5 to 63.3) among children 5 to 7 years of age and 16.6% (95% CI, -4.2 to 33.2) among those 8 to 11 years of age. Among adolescents, the overall effectiveness of the 30-μg primary vaccine series against infection with the omicron variant was 30.6% (95% CI, 26.9 to 34.1), but many adolescents had been vaccinated months earlier. Effectiveness waned over time since receipt of the second dose. Effectiveness was 35.6% (95% CI, 31.2 to 39.6) among adolescents 12 to 14 years of age and 20.9% (95% CI, 13.8 to 27.4) among those 15 to 17 years of age. In the pre-omicron study, the overall effectiveness of the 30-μg primary vaccine series against SARS-CoV-2 infection among adolescents was 87.6% (95% CI, 84.0 to 90.4) and waned relatively slowly after receipt of the second dose.
Conclusions: Vaccination in children was associated with modest, rapidly waning protection against omicron infection. Vaccination in adolescents was associated with stronger, more durable protection, perhaps because of the larger antigen dose. (Funded by Weill Cornell Medicine-Qatar and others.).
Copyright © 2022 Massachusetts Medical Society.
Figures

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
