Factor VIII mutated with Lys1813Ala within the factor IXa-binding region enhances intrinsic coagulation potential
- PMID: 36322904
- PMCID: PMC10139940
- DOI: 10.1182/bloodadvances.2022008187
Factor VIII mutated with Lys1813Ala within the factor IXa-binding region enhances intrinsic coagulation potential
Abstract
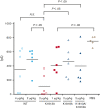
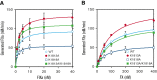
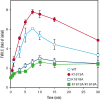
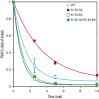
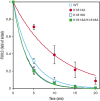
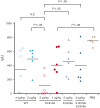
Factor VIII (FVIII) functions as a cofactor of FIXa for FX activation in the intrinsic tenase complex. The 1811-1818 region in the FVIII A3 domain was observed to contribute to FIXa binding, and the K1813A/K1818A mutant increased the binding affinity for FIXa. The current study aims to identify mutated FVIII protein(s) that increase FVIIIa cofactor activity in the 1811-1818 region. FVIII mutants with K1813A, K1818A, and K1813A/K1818A were expressed in baby hamster kidney cells and were followed by assessments using purified and global coagulation assays for mouse models with hemophilia A (HA). A surface plasmon resonance-based assay revealed that the Kd value of FVIII-K1813A for FIXa interaction was lower than that of the wild-type (WT) (3.9±0.7/6.3±0.3 nM). However, the Km value of FVIII-K1813A for FIXa on tenase activity was comparable with that of the WT, whereas the kcat of this mutant was significantly greater than that of the WT. Thrombin-catalyzed FVIII-K1813A activation was ∼1.3-fold more enhanced than that of the WT, and the spontaneous decay of activated FVIII-K1813A was ∼2.5-fold slower than that of WT. The heat stability assay revealed that the decay rate of FVIII-K1813A was ∼2.5-fold slower than that of WT. Thrombin generation assay and rotational thromboelastometry using blood samples from patients with HA demonstrated that the addition of FVIII-K1813A (0.5 nM) exhibited a coagulation potential compatible with that of WT (1 nM). In the tail clip assay of HA mice, FVIII-K1813A showed a two- to fourfold higher hemostatic potential than that of the WT. FVIII-K1813A, with higher a FIXa binding affinity, enhances the global coagulation potential because of the stability of FVIII/FVIIIa molecules.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: Y.N. and K.N. received a grant from Takeda Pharmaceutical Co. A.O. received a grant from Sanofi. N.S. taught the course endowed by CSL Behring. M.T. declares no competing financial interests.
Figures

References
-
- Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1–16. - PubMed
-
- Wood WI, Capon DJ, Simonsen CC, et al. Expression of active human factor VIII from recombinant DNA clones. Nature. 1984;312(5992):330–337. - PubMed
-
- Vehar GA, Keyt B, Eaton D, et al. Structure of human factor VIII. Nature. 1984;312(5992):337–342. - PubMed
-
- Fay PJ, Anderson MT, Chavin SI, Marder VJ. The size of human factor VIII heterodimers and the effects produced by thrombin. Biochim Biophys Acta. 1986;871(3):268–278. - PubMed
-
- Eaton D, Rodriguez H, Vehar GA. Proteolytic processing of human factor VIII. Correlation of specific cleavages by thrombin, factor Xa, and activated protein C with activation and inactivation of factor VIII coagulant activity. Biochemistry. 1986;25(2):505–512. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

