Therapeutic activity of GARP:TGF-β1 blockade in murine primary myelofibrosis
- PMID: 36322928
- PMCID: PMC10651781
- DOI: 10.1182/blood.2022017097
Therapeutic activity of GARP:TGF-β1 blockade in murine primary myelofibrosis
Abstract
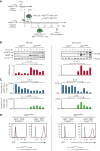
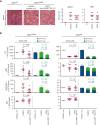
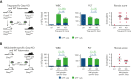
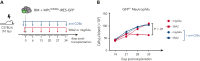
Primary myelofibrosis (PMF) is a myeloproliferative neoplasm characterized by the clonal expansion of myeloid cells, notably megakaryocytes (MKs), and an aberrant cytokine production leading to bone marrow (BM) fibrosis and insufficiency. Current treatment options are limited. TGF-β1, a profibrotic and immunosuppressive cytokine, is involved in PMF pathogenesis. While all cell types secrete inactive, latent TGF-β1, only a few activate the cytokine via cell type-specific mechanisms. The cellular source of the active TGF-β1 implicated in PMF is not known. Transmembrane protein GARP binds and activates latent TGF-β1 on the surface of regulatory T lymphocytes (Tregs) and MKs or platelets. Here, we found an increased expression of GARP in the BM and spleen of mice with PMF and tested the therapeutic potential of a monoclonal antibody (mAb) that blocks TGF-β1 activation by GARP-expressing cells. GARP:TGF-β1 blockade reduced not only fibrosis but also the clonal expansion of transformed cells. Using mice carrying a genetic deletion of Garp in either Tregs or MKs, we found that the therapeutic effects of GARP:TGF-β1 blockade in PMF imply targeting GARP on Tregs. These therapeutic effects, accompanied by increased IFN-γ signals in the spleen, were lost upon CD8 T-cell depletion. Our results suggest that the selective blockade of TGF-β1 activation by GARP-expressing Tregs increases a CD8 T-cell-mediated immune reaction that limits transformed cell expansion, providing a novel approach that could be tested to treat patients with myeloproliferative neoplasms.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: Patents pertaining to blocking antibodies against human GARP:TGF-β1 have been filed under the Patent Cooperation Treaty (International application Number PCT/IB2019/053753) with S. Lucas and P.G.C. as inventors and UCLouvain as applicant. The anti–mouse GARP:TGF-β1 antibody (clone 58A2) used herein is a surrogate for these antibodies. The laboratory of S. Lucas received research funding from AbbVie which licensed the anti-human GARP:TGF-β1 antibodies. The remaining authors declare no competing financial interests.
Figures



References
-
- Szuber N, Mudireddy M, Nicolosi M, et al. 3023 Mayo Clinic patients with myeloproliferative neoplasms: risk-stratified comparison of survival and outcomes data among disease subgroups. Mayo Clin Proc. 2019;94(4):599–610. - PubMed
-
- Cahu X, Constantinescu SN. Oncogenic drivers in myeloproliferative neoplasms: from JAK2 to calreticulin mutations. Curr Hematol Malig Rep. 2015;10(4):335–343. - PubMed
-
- Harrison C, Kiladjian JJ, Al-Ali HK, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366(9):787–798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

