Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities
- PMID: 36322929
- PMCID: PMC9936308
- DOI: 10.1182/blood.2021011994
Bispecific antibodies for the treatment of B-cell lymphoma: promises, unknowns, and opportunities
Abstract
Treatment paradigms for B-cell non-Hodgkin lymphomas (B-NHL) have shifted dramatically in the last 2 decades following the introduction of highly active immunotherapies such as rituximab. Since then, the field has continued to witness tremendous progress with the introduction of newer, more potent immunotherapeutics, including chimeric antigen receptor T-cell therapy, which have received regulatory approval for and currently play a significant role in the treatment of these diseases. Bispecific antibodies (BsAb) are a novel class of off-the-shelf T-cell redirecting drugs and are among the most promising immunotherapeutics for lymphoma today. BsAb may target various cell-surface antigens and exist in different formats. Anti-CD20xCD3 BsAb have demonstrated remarkable single-agent activity in patients with heavily pretreated B-NHL with a manageable toxicity profile dominated by T-cell overactivation syndromes. Much work remains to be done to define the optimal setting in which to deploy these drugs for B-NHL treatment, their ideal combination partners, strategies to minimize toxicity, and, perhaps most importantly, pharmacodynamic biomarkers of response and resistance. In this review, we provide an update on BsAb development in B-NHL, from discovery to clinical applications, highlighting the achievements, limitations, and future directions of the field.
© 2023 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: L.F serves as a consultant for Genmab, AbbVie, and Hoffmann-La Roche/Genentech; has received research funding from Genmab, AbbVie, and Hoffmann-La Roche/Genetech; and has membership on advisory committees of ADC Therapeutics. S.A.V. has membership on advisory committees of Immunai Inc and receives consulting fees from Koch Disruptive Industries. G.A.S. has membership on advisory committees receives consulting fees from AbbVie, Bayer, Beigene, BMS/Celgene, Epizyme, Hoffmann-La Roche/Genetech, Genmab, Incyte, Janssen, Kite/Gilead, Loxo, Miltenyi, Molecular Partners, Morphosys, Nordic Nanovector, Novartis, Rapt, Regeneron, and Takeda; and is a shareholder in Owkin.
Figures
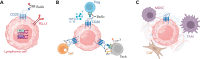
References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106(12):3725–3732. - PubMed
-
- Maloney DG. Anti-CD20 antibody therapy for B-cell lymphomas. N Engl J Med. 2012;366(21):2008–2016. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

