Hydroxyurea for secondary stroke prevention in children with sickle cell anemia in Nigeria: a randomized controlled trial
- PMID: 36322937
- PMCID: PMC10023719
- DOI: 10.1182/blood.2022016620
Hydroxyurea for secondary stroke prevention in children with sickle cell anemia in Nigeria: a randomized controlled trial
Abstract
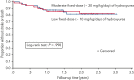
We tested the hypothesis that fixed oral moderate-dose hydroxyurea (20 mg/kg per day) for initial treatment of secondary stroke prevention results in an 80% relative risk reduction of stroke or death when compared with fixed oral low-dose hydroxyurea (10 mg/kg per day) in a phase 3 double-blind, parallel-group, randomized controlled trial in children with sickle cell anemia (SCA) living in Nigeria. A total of 101 participants were randomly allocated to low-dose (n = 49) and moderate-dose (n = 52) hydroxyurea treatment groups. The median participant follow-up was 1.6 years (interquartile range, 1.0-2.3), with a planned minimum follow-up of 3.0 years. A total of 6 recurrent strokes and 2 deaths vs 5 recurrent strokes and 3 deaths occurred in the low- and moderate-dose groups, respectively. The incidence rate ratio (IRR) of the primary outcome measure of stroke or death in the low- and moderate-dose hydroxyurea treatment groups was 0.98 (95% confidence interval [CI], 0.32-3.00; P = .97). The trial was stopped early owing to no clinical difference in the incidence rates of the primary outcome measure. The incidence rates of recurrent strokes were 7.1 and 6.0 per 100 person-years in the low- and moderate-dose groups, respectively, (IRR, 1.18; 95% CI, 0.30-4.88; P = .74). As a measure of adherence to the oral hydroxyurea therapy, the median percent of returned pills was 3.0% and 2.6% in the low- and moderate-dose groups, respectively. No participant had hydroxyurea therapy stopped for myelosuppression. For children with SCA in low-income settings without access to regular blood transfusion therapy, initial low-dose hydroxyurea is a minimum known efficacious dose for secondary stroke prevention.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: M.R.D. and his institution are the sponsors of 2 externally funded research investigator-initiated projects; Global Blood Therapeutics will provide funding for these clinical studies but will not be a cosponsor of either study; he is not receiving any compensation for the conduct of these 2 investigator-initiated observational studies; he is a member of the Global Blood Therapeutics advisory board for a proposed randomized controlled trial, for which he receives compensation; he is on the steering committee for a Novartis-sponsored phase 2 trial to prevent priapism in men; he was a medical adviser for the development of the CTX001 Early Economic Model; he provided medical input on the economic model as part of an expert reference group for Vertex/CRISPR CTX001 Early Economic Model in 2020; and he provided a consultation to the Forma Pharmaceutical company about sickle cell disease from 2021 to 2022. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Hydroxyurea: how much is enough?Blood. 2023 Feb 23;141(8):813-814. doi: 10.1182/blood.2022018790. Blood. 2023. PMID: 36821183 No abstract available.
References
-
- Lagunju IA, Brown BJ, Sodeinde OO. Chronic blood transfusion for primary and secondary stroke prevention in Nigerian children with sickle cell disease: a 5-year appraisal. Pediatr Blood Cancer. 2013;60(12):1940–1945. - PubMed
-
- Patel DK, Mashon RS, Patel S, Das BS, Purohit P, Bishwal SC. Low dose hydroxyurea is effective in reducing the incidence of painful crisis and frequency of blood transfusion in sickle cell anemia patients from eastern India. Hemoglobin. 2012;36(5):409–420. - PubMed
-
- Svarch E, Machín S, Nieves RM, Mancia de Reyes AG, Navarrete M, Rodríguez H. Hydroxyurea treatment in children with sickle cell anemia in Central America and the Caribbean countries. Pediatr Blood Cancer. 2006;47(1):111–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

