The past, present, and future physiology and pharmacology of glucagon
- PMID: 36323234
- PMCID: PMC9641554
- DOI: 10.1016/j.cmet.2022.10.001
The past, present, and future physiology and pharmacology of glucagon
Abstract
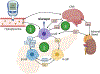
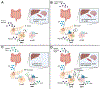
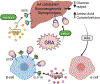
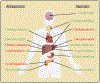
The evolution of glucagon has seen the transition from an impurity in the preparation of insulin to the development of glucagon receptor agonists for use in type 1 diabetes. In type 2 diabetes, glucagon receptor antagonists have been explored to reduce glycemia thought to be induced by hyperglucagonemia. However, the catabolic actions of glucagon are currently being leveraged to target the rise in obesity that paralleled that of diabetes, bringing the pharmacology of glucagon full circle. During this evolution, the physiological importance of glucagon advanced beyond the control of hepatic glucose production, incorporating critical roles for glucagon to regulate both lipid and amino acid metabolism. Thus, it is unsurprising that the study of glucagon has left several paradoxes that make it difficult to distill this hormone down to a simplified action. Here, we describe the history of glucagon from the past to the present and suggest some direction to the future of this field.
Keywords: diabetes; glucagon; glucose; insulin; α cells.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Our group receives financial support from Eli Lilly and Novo Nordisk to carry out basic science in this area. J.E.C. and D.A.D’A. serve as advisors for Structure Therapeutics. D.A.D’A. serves as an advisor for Eli Lilly.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

