Spatiotemporal and genetic regulation of A-to-I editing throughout human brain development
- PMID: 36323256
- PMCID: PMC9704047
- DOI: 10.1016/j.celrep.2022.111585
Spatiotemporal and genetic regulation of A-to-I editing throughout human brain development
Abstract
Posttranscriptional RNA modifications by adenosine-to-inosine (A-to-I) editing are abundant in the brain, yet elucidating functional sites remains challenging. To bridge this gap, we investigate spatiotemporal and genetically regulated A-to-I editing sites across prenatal and postnatal stages of human brain development. More than 10,000 spatiotemporally regulated A-to-I sites were identified that occur predominately in 3' UTRs and introns, as well as 37 sites that recode amino acids in protein coding regions with precise changes in editing levels across development. Hyper-edited transcripts are also enriched in the aging brain and stabilize RNA secondary structures. These features are conserved in murine and non-human primate models of neurodevelopment. Finally, thousands of cis-editing quantitative trait loci (edQTLs) were identified with unique regulatory effects during prenatal and postnatal development. Collectively, this work offers a resolved atlas linking spatiotemporal variation in editing levels to genetic regulatory effects throughout distinct stages of brain maturation.
Keywords: CP: Molecular biology; CP: Neuroscience; RNA modifications; RNA recoding; brain maturation; edQTLs; hyper-editing; late-fetal transition.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.S.B. is a consultant for Shape Therapeutics. J.D.B. is a consultant for BridgeBio Pharma.
Figures





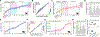
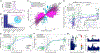
References
-
- Behm M, and Öhman M (2016). RNA editing: a contributor to neuronal dynamics in the mammalian brain. Trends Genet. 32, 165–175. - PubMed
-
- Mehler MF, and Mattick JS (2007). Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol. Rev. 87, 799–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

