Pre-infection antiviral innate immunity contributes to sex differences in SARS-CoV-2 infection
- PMID: 36323307
- PMCID: PMC9623453
- DOI: 10.1016/j.cels.2022.10.005
Pre-infection antiviral innate immunity contributes to sex differences in SARS-CoV-2 infection
Abstract
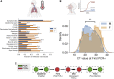
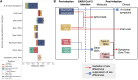
Male sex is a major risk factor for SARS-CoV-2 infection severity. To understand the basis for this sex difference, we studied SARS-CoV-2 infection in a young adult cohort of United States Marine recruits. Among 2,641 male and 244 female unvaccinated and seronegative recruits studied longitudinally, SARS-CoV-2 infections occurred in 1,033 males and 137 females. We identified sex differences in symptoms, viral load, blood transcriptome, RNA splicing, and proteomic signatures. Females had higher pre-infection expression of antiviral interferon-stimulated gene (ISG) programs. Causal mediation analysis implicated ISG differences in number of symptoms, levels of ISGs, and differential splicing of CD45 lymphocyte phosphatase during infection. Our results indicate that the antiviral innate immunity set point causally contributes to sex differences in response to SARS-CoV-2 infection. A record of this paper's transparent peer review process is included in the supplemental information.
Keywords: COVID-19; SARS-CoV-2; alternative splicing; causal mediation; differential expression; interferon-stimulated genes; sex differences; viral infection.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests C.W.G., R.A.L., S.E.L., M.A.S., M.S.T., D.L.W., and A.G.L. are military service members or government service employees. This work was prepared as part of their official duties. Title 17, US Code §105 provides that copyright protection under this title is not available for any work of the US Government. Title 17, US code §101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties. The views expressed in the article are those of the authors and do not necessarily express the official policy and position of the US Navy, the Department of Defense, and the US Government or the institutions affiliated with the authors. O.G.T. is on the advisory board of Cell Systems.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

