Cost-Effectiveness of Dapagliflozin as a Treatment for Chronic Kidney Disease: A Health-Economic Analysis of DAPA-CKD
- PMID: 36323444
- PMCID: PMC9718008
- DOI: 10.2215/CJN.03790322
Cost-Effectiveness of Dapagliflozin as a Treatment for Chronic Kidney Disease: A Health-Economic Analysis of DAPA-CKD
Abstract
Background and objectives: CKD imposes a significant burden on patients and health care providers, particularly upon reaching kidney failure when patients may require KRT. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial demonstrated that dapagliflozin, with standard therapy, reduced CKD progression and KRT requirement. The study objective was to estimate the cost-effectiveness of dapagliflozin for the treatment of CKD from payer perspectives in the United Kingdom, Germany, and Spain.
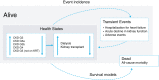
Design, setting, participants, & measurements: We constructed a lifetime Markov model to characterize outcomes in patients with CKD on the basis of the DAPA-CKD trial. Health states were defined by eGFR level and KRT type. Direct health care costs and utility values were sourced from published literature and the DAPA-CKD trial, respectively. Costs and benefits were discounted at 3.5% per annum in the United Kingdom and 3% in Germany and Spain.
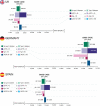
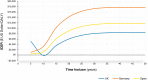
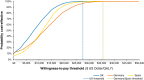
Results: In patients eligible for the DAPA-CKD trial, treatment with dapagliflozin was predicted to reduce rates of CKD progression, with patients predicted to spend 1.7 (95% credibility interval, 0.8 to 2.4) more years in the eGFR range 15-89 ml/min per 1.73 m2 versus standard therapy alone (12.1; 95% credibility interval, 8.9 to 14.1 versus 10.4; 95% credibility interval, 7.7 to 12.4 years). Life expectancy (undiscounted) was correspondingly predicted to increase by 1.7 (95% credibility interval, 0.7 to 2.5) years (15.5; 95% credibility interval, 11.1 to 18.2 versus 13.8; 95% credibility interval, 9.9 to 16.5 years). This in addition to reduced incidence of adverse clinical outcomes, including hospitalization for heart failure, resulted in modeled quality-adjusted life year (discounted) gains between 0.82 (95% credibility interval, 0.38 to 1.18) and 1.00 (95% credibility interval, 0.46 to 1.41). These gains translated to incremental cost-effectiveness ratios of $8280, $17,623, and $11,687 in the United Kingdom, Germany, and Spain, respectively, indicating cost-effectiveness at willingness-to-pay thresholds (United Kingdom: $27,510 per quality-adjusted life year; Germany and Spain: $35,503 per quality-adjusted life year).
Conclusions: In patients meeting the eligibility requirements for the DAPA-CKD trial, dapagliflozin is likely to be a cost-effective treatment within the UK, German, and Spanish health care systems.
Clinical trial registry name and registration number: Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD), NCT03036150.
Keywords: SGLT2 inhibitor; chronic kidney disease; cost-effectiveness; dapagliflozin.
Copyright © 2022 by the American Society of Nephrology.
Figures

Comment in
-
A Blueprint for Assessing Affordability of SGLT2 Inhibitors in the United States: The Cost-Effectiveness of Dapagliflozin in Three European Countries.Clin J Am Soc Nephrol. 2022 Dec;17(12):1707-1709. doi: 10.2215/CJN.09900822. Epub 2022 Nov 2. Clin J Am Soc Nephrol. 2022. PMID: 36323445 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

