Insight into the Photodynamics of Photostabilizer Molecules
- PMID: 36323639
- PMCID: PMC9677431
- DOI: 10.1021/acs.jpca.2c05580
Insight into the Photodynamics of Photostabilizer Molecules
Abstract
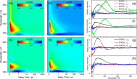
Solar exposure of avobenzone, one of the most widely used commercial UVA filters on the market, is known to cause significant degradation. This finding has fueled research into developing photostabilizer molecules. In an effort to provide insight into their stand-alone photoprotection properties, the excited state dynamics of the photostabilizer, 3-(3,4,5-trimethoxybenzylidene) pentane-2,4-dione (TMBP), and its phenolic derivative, 3-(4-hydroxy-3,5-dimethoxybenzylidene) pentane-2,4-dione (DMBP), were studied with ultrafast transient absorption spectroscopy. Solutions of TMPB and DMBP in ethanol and in an industry-standard emollient, as well as TMBP and DMBP deposited on synthetic skin mimic, were investigated. These experiments were allied with computational methods to aid interpretation of the experimental data. Upon photoexcitation, these photostabilizers repopulate the electronic ground state via nonradiative decay within a few picoseconds involving a twisted intramolecular charge transfer configuration in the excited state, followed by internal conversion and subsequent vibrational cooling in the ground state. This finding implies that, aside from acting as a photostabilizer to certain UV filters, TMBP and DMBP may offer additional photoprotection in a sunscreen formulation as a stand-alone UV filter. Finally, TMBP and DMBP could also find applications as molecular photon-to-heat converters.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Dahle J.; Kvam E. Induction of delayed mutations and chromosomal instability in fibroblasts after UVA-, UVB-, and X-radiation. Cancer Res. 2003, 63 (7), 1464–1469. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

