A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability
- PMID: 36323666
- PMCID: PMC9628444
- DOI: 10.1038/s41541-022-00549-y
A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability
Erratum in
-
Author Correction: A self-amplifying RNA vaccine against COVID-19 with long-term room-temperature stability.NPJ Vaccines. 2022 Nov 23;7(1):150. doi: 10.1038/s41541-022-00578-7. NPJ Vaccines. 2022. PMID: 36418864 Free PMC article. No abstract available.
Abstract
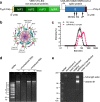
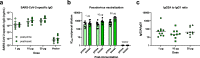
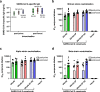
mRNA vaccines were the first to be authorized for use against SARS-CoV-2 and have since demonstrated high efficacy against serious illness and death. However, limitations in these vaccines have been recognized due to their requirement for cold storage, short durability of protection, and lack of access in low-resource regions. We have developed an easily-manufactured, potent self-amplifying RNA (saRNA) vaccine against SARS-CoV-2 that is stable at room temperature. This saRNA vaccine is formulated with a nanostructured lipid carrier (NLC), providing stability, ease of manufacturing, and protection against degradation. In preclinical studies, this saRNA/NLC vaccine induced strong humoral immunity, as demonstrated by high pseudovirus neutralization titers to the Alpha, Beta, and Delta variants of concern and induction of bone marrow-resident antibody-secreting cells. Robust Th1-biased T-cell responses were also observed after prime or homologous prime-boost in mice. Notably, the saRNA/NLC platform demonstrated thermostability when stored lyophilized at room temperature for at least 6 months and at refrigerated temperatures for at least 10 months. Taken together, this saRNA delivered by NLC represents a potential improvement in RNA technology that could allow wider access to RNA vaccines for the current COVID-19 and future pandemics.
© 2022. The Author(s).
Conflict of interest statement
A.G., C.B.F., C.J.P., E.A.V., and P.S.S. declare no competing non-financial interests but the following competing financial interests. C.B.F. is co-inventor on patent applications relating to PCT/US2018/37783, “Nanostructured Lipid Carriers and stable emulsions and uses thereof.” A.G. and E.A.V. are co-inventors on U.S. patent application nos. PCT/US21/40388, “Co-lyophilized RNA and Nanostructured Lipid Carrier,” and 63/144,169, “A thermostable, flexible RNA vaccine delivery platform for pandemic response.” C.J.P. owns shares and possesses stock options in Amyris, Inc. P.S.S. owns shares of ImmunityBio, Inc. All other authors declare they have no competing interests.
Figures



References
-
- Mathieu E, et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

