Extracellular fluid viscosity enhances cell migration and cancer dissemination
- PMID: 36323783
- PMCID: PMC9646524
- DOI: 10.1038/s41586-022-05394-6
Extracellular fluid viscosity enhances cell migration and cancer dissemination
Abstract
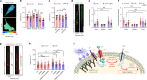
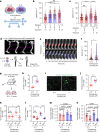
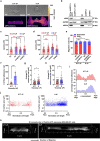
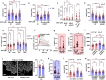
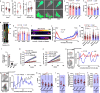
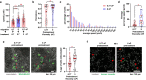
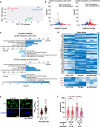
Cells respond to physical stimuli, such as stiffness1, fluid shear stress2 and hydraulic pressure3,4. Extracellular fluid viscosity is a key physical cue that varies under physiological and pathological conditions, such as cancer5. However, its influence on cancer biology and the mechanism by which cells sense and respond to changes in viscosity are unknown. Here we demonstrate that elevated viscosity counterintuitively increases the motility of various cell types on two-dimensional surfaces and in confinement, and increases cell dissemination from three-dimensional tumour spheroids. Increased mechanical loading imposed by elevated viscosity induces an actin-related protein 2/3 (ARP2/3)-complex-dependent dense actin network, which enhances Na+/H+ exchanger 1 (NHE1) polarization through its actin-binding partner ezrin. NHE1 promotes cell swelling and increased membrane tension, which, in turn, activates transient receptor potential cation vanilloid 4 (TRPV4) and mediates calcium influx, leading to increased RHOA-dependent cell contractility. The coordinated action of actin remodelling/dynamics, NHE1-mediated swelling and RHOA-based contractility facilitates enhanced motility at elevated viscosities. Breast cancer cells pre-exposed to elevated viscosity acquire TRPV4-dependent mechanical memory through transcriptional control of the Hippo pathway, leading to increased migration in zebrafish, extravasation in chick embryos and lung colonization in mice. Cumulatively, extracellular viscosity is a physical cue that regulates both short- and long-term cellular processes with pathophysiological relevance to cancer biology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Comment in
-
The memory of viscosity.Nat Rev Mol Cell Biol. 2023 Jan;24(1):3. doi: 10.1038/s41580-022-00563-x. Nat Rev Mol Cell Biol. 2023. PMID: 36380159 No abstract available.
References
-
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

