Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era
- PMID: 36324319
- PMCID: PMC9619560
- DOI: 10.1093/ofid/ofac517
Evaluation of Bebtelovimab for Treatment of Covid-19 During the SARS-CoV-2 Omicron Variant Era
Abstract
Background: Monoclonal antibody (mAb) treatment is associated with decreased risk of hospitalization and death in high-risk outpatients with mild to moderate coronavirus disease 2019 (COVID-19) caused by early severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants. Bebtelovimab exhibits in vitro activity against the Omicron variant and its sublineages; however, clinical data are lacking.
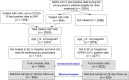
Methods: A retrospective cohort study was conducted comparing bebtelovimab-treated patients with propensity score-adjusted and matched nontreated control groups. Participants included high-risk outpatients eligible for bebtelovimab treatment under Emergency Use Authorization with a positive SARS-CoV-2 test from March 30 to May 28, 2022. Treated patients received single-dose intravenous treatment with bebtelovimab. The primary outcome was hospitalization or death over 28 days.
Results: Before matching/statistical adjustment, mAb-treated patients were, on average, 10 years older than nontreated patients (61.6 vs 51.3 years) and had higher prevalence of obstructive sleep apnea, hypertension, chronic kidney disease, cancer, organ or cell transplant, and immunocompromised status (standardized mean differences ≥0.20). The adjusted odds ratio (OR) of hospitalization or death comparing 1006 treated with 2023 nontreated patients was 0.50 (95% CI, 0.31-0.80). Among 930 treated and 930 propensity score-matched nontreated patients, the incidence of hospitalization or death was 3.1% vs 5.5%, respectively (conditional OR, 0.53; 95% CI, 0.32-0.86). The lower odds ratio of hospitalization or death associated with bebtelovimab treatment was most evident in older patients, those with immunocompromised status, and fully vaccinated patients.
Conclusions: Monoclonal antibody treatment with bebtelovimab among COVID-19 outpatients is associated with lower odds of hospitalization or death, particularly among immunocompromised and older patients.
Keywords: Omicron SARS-CoV-2 variant; bebtelovimab; death; hospitalization; immunosuppression; propensity score matching.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Gupta A, Gonzalez-Rojas Y, Juarez E, et al. . Early treatment for COVID-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med 2021; 385:1941–50. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous