mRNA Vaccination Decreases COVID-19-Associated Morbidity and Mortality Among Organ Transplant Recipients: A Contemporary Cohort Study
- PMID: 36324327
- PMCID: PMC9619466
- DOI: 10.1093/ofid/ofac503
mRNA Vaccination Decreases COVID-19-Associated Morbidity and Mortality Among Organ Transplant Recipients: A Contemporary Cohort Study
Abstract
Background: Organ transplant recipients (OTRs) are less protected from vaccination than immunocompetent hosts. Additional vaccine doses have shown increased immunogenicity. Few studies have assessed their clinical efficacy, particularly against Omicron variants, as most included patients from earlier phases of the pandemic, with higher base mortality rates.
Methods: We studied adult OTRs who had coronavirus disease 2019 (COVID-19) between 12/15/21 and 5/25/22. We compared clinical outcomes between those who had received 2 or ≥3 doses of an mRNA vaccine and concurrent unvaccinated controls.
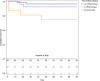
Results: Among 103 OTRs, vaccination was associated with lower 90-day mortality (unvaccinated vs 2 vs ≥3 doses: 25% vs 7% vs 3%; P = .003), hospital (unvaccinated vs 2 vs ≥3 doses: 56% vs 37% vs 27%; P = .018) and intensive care unit (ICU; unvaccinated vs 2 vs ≥3 doses: 25% vs 15% vs 3%; P = .001) admission rates, and peak O2 requirements (ordinal scale Kendall's tau b = -0.309 [lower scores, ie, O2 requirements with more vaccine doses]; P = .003). Age (age >60 years: adjusted hazard ratio [aHR], 7.73; P = .016; administration of antispike monoclonal antibody: aHR, 0.17; P = .042) and vaccination, especially with ≥3 doses (aHR, 0.105; P = .01), were independently associated with 90-day mortality. Black (P = .021) and Hispanic (P = .016) OTRs were underrepresented among the vaccinated, especially in the ≥3-dose group.
Conclusions: Despite lower mRNA vaccine efficacy in OTRs and against Omicron variants, vaccination protects this vulnerable patient population from severe COVID-19 and death. Ethnic and racial disparities in health care have been exacerbated by the COVID-19 pandemic and warrant better community outreach efforts.
Keywords: COVID-19; Omicron; SARS-CoV-2; infection; transplant; vaccines.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. D.F. has received research support from Viracor, Astellas, and Merck and a consultant fee from Viracor. All other authors have nothing to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Saharia KK, Anjan S, Streit J, et al. . Clinical characteristics of COVID-19 in solid organ transplant recipients following COVID-19 vaccination: a multicenter case series. Transpl Infect Dis 2022; 24:e13774. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

