Encoding type, medication, and deep brain stimulation differentially affect memory-guided sequential reaching movements in Parkinson's disease
- PMID: 36324383
- PMCID: PMC9618698
- DOI: 10.3389/fneur.2022.980935
Encoding type, medication, and deep brain stimulation differentially affect memory-guided sequential reaching movements in Parkinson's disease
Abstract
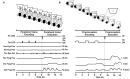
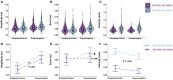
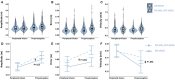
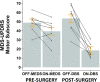
Memory-guided movements, vital to daily activities, are especially impaired in Parkinson's disease (PD). However, studies examining the effects of how information is encoded in memory and the effects of common treatments of PD, such as medication and subthalamic nucleus deep brain stimulation (STN-DBS), on memory-guided movements are uncommon and their findings are equivocal. We designed two memory-guided sequential reaching tasks, peripheral-vision or proprioception encoded, to investigate the effects of encoding type (peripheral-vision vs. proprioception), medication (on- vs. off-), STN-DBS (on- vs. off-, while off-medication), and compared STN-DBS vs. medication on reaching amplitude, error, and velocity. We collected data from 16 (analyzed n = 7) participants with PD, pre- and post-STN-DBS surgery, and 17 (analyzed n = 14) healthy controls. We had four important findings. First, encoding type differentially affected reaching performance: peripheral-vision reaches were faster and more accurate. Also, encoding type differentially affected reaching deficits in PD compared to healthy controls: peripheral-vision reaches manifested larger deficits in amplitude. Second, the effect of medication depended on encoding type: medication had no effect on amplitude, but reduced error for both encoding types, and increased velocity only during peripheral-vision encoding. Third, the effect of STN-DBS depended on encoding type: STN-DBS increased amplitude for both encoding types, increased error during proprioception encoding, and increased velocity for both encoding types. Fourth, STN-DBS was superior to medication with respect to increasing amplitude and velocity, whereas medication was superior to STN-DBS with respect to reducing error. We discuss our findings in the context of the previous literature and consider mechanisms for the differential effects of medication and STN-DBS.
Keywords: Parkinson's disease; encoding; peripheral-vision levodopa; proprioception; subthalamic nucleus deep brain stimulation (STN-DBS).
Copyright © 2022 David, Rivera, Entezar, Arora, Drane, Munoz, Rosenow, Sani, Pal, Verhagen-Metman and Corcos.
Conflict of interest statement
Authors FD and MM received grant support from NIH. Author JR consults for Boston Scientific. Author SS received grant support from NIH, Medtronic, Abbott, and Boston Scientific. Author GP received grant support from NIH and the Parkinson's Disease Foundation. Author LV-M receives honoraria for consulting services/advisory boards from AbbVie, Abbott, Avion, and research support from AbbVie, Abbott, Biogen, Boston Sci., Chase, Medtronic, Neuroderm, Addex, UCB, and NIH. Author DC received grant support from NIH and Michael J. Fox and receives lecture and reviewer fees from NIH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Goldberg G. Supplementary motor area structure and function - review and hypotheses. Behav Brain Sci. (1985) 8:567–88. 10.1017/S0140525x00045167 - DOI
-
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Internal vs external generation of movements: Differential neural pathways involved in bimanual coordination performed in the presence or absence of augmented visual feedback. Neuroimage. (2003) 19:764–76. 10.1016/s1053-8119(03)00148-4 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

