Neutrophils in pancreatic cancer: Potential therapeutic targets
- PMID: 36324574
- PMCID: PMC9618950
- DOI: 10.3389/fonc.2022.1025805
Neutrophils in pancreatic cancer: Potential therapeutic targets
Abstract
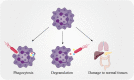
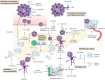
Pancreatic cancer is a digestive system malignancy and poses a high mortality worldwide. Traditionally, neutrophils have been thought to play a role in acute inflammation. In contrast, their importance during tumor diseases has been less well studied. Generally, neutrophils are recruited into the tumor microenvironment and exert inflammation and tumor-promoting effects. As an essential part of the tumor microenvironment, neutrophils play diverse roles in pancreatic cancer, such as angiogenesis, progression, metastasis and immunosuppression. Additionally, neutrophils can be a new potential therapeutic target in cancer. Inhibitors of cytokines, chemokines and neutrophil extracellular traps can exert antitumor effects. In this review, we describe the role of neutrophils in the development and progression of pancreatic cancer, discuss their potential as therapeutic targets, and aim to provide ideas for improving the prognosis of patients with this malignant tumor disease.
Keywords: anticancer therapy; neutrophil; neutrophil extracellular trap; pancreatic cancer; tumor-associated neutrophil.
Copyright © 2022 Jiang, Li, Xiang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Neutrophils in the Tumor Microenvironment.Adv Exp Med Biol. 2020;1224:1-20. doi: 10.1007/978-3-030-35723-8_1. Adv Exp Med Biol. 2020. PMID: 32036601 Free PMC article. Review.
-
Tumor-associated neutrophils in pancreatic cancer progression and metastasis.Am J Cancer Res. 2023 Dec 15;13(12):6176-6189. eCollection 2023. Am J Cancer Res. 2023. PMID: 38187037 Free PMC article. Review.
-
Tumor-Associated Neutrophils in Cancer: Going Pro.Cancers (Basel). 2019 Apr 19;11(4):564. doi: 10.3390/cancers11040564. Cancers (Basel). 2019. PMID: 31010242 Free PMC article. Review.
-
Neutrophil extracellular traps: New players in cancer research.Front Immunol. 2022 Aug 19;13:937565. doi: 10.3389/fimmu.2022.937565. eCollection 2022. Front Immunol. 2022. PMID: 36059520 Free PMC article. Review.
-
Neutrophil extracellular traps in cancer.Semin Cancer Biol. 2022 Feb;79:91-104. doi: 10.1016/j.semcancer.2021.07.011. Epub 2021 Jul 16. Semin Cancer Biol. 2022. PMID: 34280576 Review.
Cited by
-
An integrated overview of the immunosuppression features in the tumor microenvironment of pancreatic cancer.Front Immunol. 2023 Sep 12;14:1258538. doi: 10.3389/fimmu.2023.1258538. eCollection 2023. Front Immunol. 2023. PMID: 37771596 Free PMC article. Review.
-
The Efficacy of Targeted Monoclonal IgA Antibodies Against Pancreatic Ductal Adenocarcinoma.Cells. 2025 Apr 24;14(9):632. doi: 10.3390/cells14090632. Cells. 2025. PMID: 40358156 Free PMC article.
-
Tumor-educated cells in tumor microenvironment: Key drivers of immunotherapy resistance.Chin J Cancer Res. 2025 Jun 30;37(3):446-465. doi: 10.21147/j.issn.1000-9604.2025.03.12. Chin J Cancer Res. 2025. PMID: 40642484 Free PMC article.
-
Combined impact of prognostic nutritional index, fibrinogen-to-albumin ratio, and neutrophil-to-lymphocyte ratio on surgical outcomes and prognosis in hepatocellular carcinoma.Am J Cancer Res. 2025 Feb 15;15(2):439-451. doi: 10.62347/RTMF3105. eCollection 2025. Am J Cancer Res. 2025. PMID: 40084351 Free PMC article.
-
Clinical significance of FBXO43 in hepatocellular carcinoma and its impact on tumor cell proliferation, migration and invasion.PeerJ. 2023 May 22;11:e15373. doi: 10.7717/peerj.15373. eCollection 2023. PeerJ. 2023. PMID: 37250703 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

