Osimertinib versus platinum-pemetrexed in patients with previously treated EGFR T790M advanced non-small cell lung cancer: An updated AURA3 trial-based cost-effectiveness analysis
- PMID: 36324594
- PMCID: PMC9619214
- DOI: 10.3389/fonc.2022.833773
Osimertinib versus platinum-pemetrexed in patients with previously treated EGFR T790M advanced non-small cell lung cancer: An updated AURA3 trial-based cost-effectiveness analysis
Abstract
Background: A recently overall survival (OS) analysis from the AURA3 trial indicated that osimertinib improves median OS versus platinum-pemetrexed for patients with previously treated epidermal growth factor receptor (EGFR) T790M advanced non-small cell lung cancer (NSCLC). Here, we assessed the cost-effectiveness of second-line osimertinib versus platinum-pemetrexed, from the perspectives of the United States payer and the Chinese health care system.
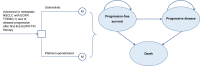
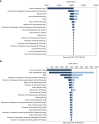
Methods: A Markov model was constructed to compare the costs and health outcomes of osimertinib versus platinum-pemetrexed in second-line treatment of EGFR T790M advanced NSCLC. Life years (LYs), quality adjusted life years (QALYs), costs, and incremental cost-effectiveness ratios (ICERs) were calculated. One-way and probabilistic sensitivity analyses assessed the robustness of the model. Cost-effectiveness was examined in the intention-to-treat (ITT) population and central nervous system (CNS) metastases population.
Results: In the United States, compared with platinum-pemetrexed, osimertinib yielded additional effectiveness of 0.43 QALYs and -0.12 QALYs, with incremental costs of $67,588 and $16,465 in the ITT population and CNS metastases population, respectively. The ICERs of osimertinib over platinum-pemetrexed were $159,126/QALY and $-130,830/QALY, respectively. The probability of osimertinib being cost-effective was 37% and 5.76%, respectively, at the willingness-to-pay (WTP) threshold of $150,000/QALY. In China, osimertinib showed incremental effectiveness of 0.34 QALYs and -0.14 QALYs, with incremental costs of $1,663 and $-505, resulting in ICERs of $4,950/QALY and $3,754/QALY in the ITT population and CNS metastases population, respectively. At the WTP threshold of $37,489/QALY, there was a 100% and 26% likelihood that osimertinib was cost-effective in the ITT population and CNS metastases population.
Conclusion: In the United States, second-line osimertinib treatment for EGFR T790M advanced NSCLC is not cost-effective compared to platinum-pemetrexed under the current WTP threshold. When the osimertinib price reduces, the economic outcome may become favorable. In China, assuming a WTP threshold of $37,489/QALY, osimertinib is the dominant treatment strategy compared with platinum-pemetrexed in the ITT population and provides cost savings for CNS metastases patients.
Keywords: EGFR; cost-effectiveness; non-small-cell lung cancer; osimertinib; platinum-pemetrexed.
Copyright © 2022 Shi, Pei and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cost-Effectiveness of Osimertinib for EGFR Mutation-Positive Non-Small Cell Lung Cancer after Progression following First-Line EGFR TKI Therapy.J Thorac Oncol. 2018 Feb;13(2):184-193. doi: 10.1016/j.jtho.2017.10.012. Epub 2017 Oct 31. J Thorac Oncol. 2018. PMID: 29101057
-
Cost-effectiveness of Osimertinib as a Second-line Treatment in Patients With EGFR-mutated Advanced Non-Small Cell Lung Cancer in China.Clin Ther. 2019 Nov;41(11):2308-2320.e11. doi: 10.1016/j.clinthera.2019.09.008. Epub 2019 Oct 10. Clin Ther. 2019. PMID: 31607559
-
Cost-effectiveness analysis of different sequences of osimertinib administration for epidermal growth factor receptor-mutated non-small-cell lung cancer.Exp Ther Med. 2021 Apr;21(4):343. doi: 10.3892/etm.2021.9774. Epub 2021 Feb 10. Exp Ther Med. 2021. PMID: 33732316 Free PMC article.
-
Cost-effectiveness analyses of amivantamab plus lazertinib and lazertinib versus osimertinib in non-small cell lung cancer with EGFR mutations.Front Pharmacol. 2025 May 2;16:1527614. doi: 10.3389/fphar.2025.1527614. eCollection 2025. Front Pharmacol. 2025. PMID: 40385479 Free PMC article.
-
Osimertinib: A Review in T790M-Positive Advanced Non-Small Cell Lung Cancer.Target Oncol. 2017 Aug;12(4):555-562. doi: 10.1007/s11523-017-0519-0. Target Oncol. 2017. PMID: 28710746 Review.
Cited by
-
Real-world efficacy of adjuvant therapy for totally resected stage I lung adenocarcinoma patients with pathological high-risk factors: propensity score analysis.BMC Surg. 2024 May 8;24(1):140. doi: 10.1186/s12893-024-02428-w. BMC Surg. 2024. PMID: 38720305 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

