Photochromic spiro-indoline naphthoxazines and naphthopyrans in dye-sensitized solar cells
- PMID: 36324610
- PMCID: PMC9549531
- DOI: 10.1039/d2qm00375a
Photochromic spiro-indoline naphthoxazines and naphthopyrans in dye-sensitized solar cells
Abstract
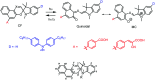
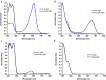
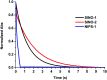
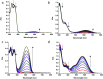
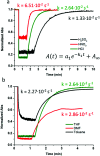
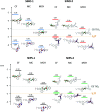
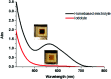
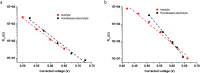
Photochromic dyes possess unique properties that can be exploited in different domains, including optics, biomedicine and optoelectronics. Herein, we explore the potential of photochromic spiro-indoline naphthoxazine (SINO) and naphthopyran (NIPS) for application in photovoltaics. We designed and synthesized four new photosensitizers with a donor-pi-acceptor structure embedding SINO and NIPS units as photochromic cores. Their optical, photochromic and acidochromic properties were thoroughly studied to establish structure-properties relationships. Then, after unravelling the possible forms adopted depending on the stimuli, their photovoltaic properties were evaluated in DSSCs. Although the photochromic behavior is not always preserved, we elucidate the interplay between photochromic, acidochromic and photovoltaic properties and we demonstrate that these dyes can act as photosensitizers in DSSCs. We report a maximum power conversion efficiency of 2.7% with a NIPS-based dye, a tenfold improvement in comparison to previous works on similar class of compounds. This work opens new perspectives of developments for SINO and NIPS in optical and photovoltaic devices, and it provides novel research directions to design photochromic materials with improved characteristics.
This journal is © the Partner Organisations.
Conflict of interest statement
There are no conflicts to declare.
Figures


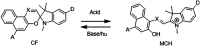


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
