Imaging Transcriptomics of Brain Disorders
- PMID: 36324650
- PMCID: PMC9616271
- DOI: 10.1016/j.bpsgos.2021.10.002
Imaging Transcriptomics of Brain Disorders
Abstract
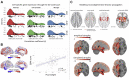
Noninvasive neuroimaging is a powerful tool for quantifying diverse aspects of brain structure and function in vivo, and it has been used extensively to map the neural changes associated with various brain disorders. However, most neuroimaging techniques offer only indirect measures of underlying pathological mechanisms. The recent development of anatomically comprehensive gene expression atlases has opened new opportunities for studying the transcriptional correlates of noninvasively measured neural phenotypes, offering a rich framework for evaluating pathophysiological hypotheses and putative mechanisms. Here, we provide an overview of some fundamental methods in imaging transcriptomics and outline their application to understanding brain disorders of neurodevelopment, adulthood, and neurodegeneration. Converging evidence indicates that spatial variations in gene expression are linked to normative changes in brain structure during age-related maturation and neurodegeneration that are in part associated with cell-specific gene expression markers of gene expression. Transcriptional correlates of disorder-related neuroimaging phenotypes are also linked to transcriptionally dysregulated genes identified in ex vivo analyses of patient brains. Modeling studies demonstrate that spatial patterns of gene expression are involved in regional vulnerability to neurodegeneration and the spread of disease across the brain. This growing body of work supports the utility of transcriptional atlases in testing hypotheses about the molecular mechanism driving disease-related changes in macroscopic neuroimaging phenotypes.
Keywords: Brain imaging; Connectome; Gene expression; Neurodegeneration; Neurodevelopment; Psychiatric disorders.
© 2021 The Authors.
Figures



Similar articles
-
A practical guide to linking brain-wide gene expression and neuroimaging data.Neuroimage. 2019 Apr 1;189:353-367. doi: 10.1016/j.neuroimage.2019.01.011. Epub 2019 Jan 12. Neuroimage. 2019. PMID: 30648605
-
Toward Best Practices for Imaging Transcriptomics of the Human Brain.Biol Psychiatry. 2023 Mar 1;93(5):391-404. doi: 10.1016/j.biopsych.2022.10.016. Epub 2022 Nov 5. Biol Psychiatry. 2023. PMID: 36725139 Review.
-
Right care, first time: a highly personalised and measurement-based care model to manage youth mental health.Med J Aust. 2019 Nov;211 Suppl 9:S3-S46. doi: 10.5694/mja2.50383. Med J Aust. 2019. PMID: 31679171
-
Bridging the Gap between Connectome and Transcriptome.Trends Cogn Sci. 2019 Jan;23(1):34-50. doi: 10.1016/j.tics.2018.10.005. Epub 2018 Nov 16. Trends Cogn Sci. 2019. PMID: 30455082 Review.
-
Where the genome meets the connectome: Understanding how genes shape human brain connectivity.Neuroimage. 2021 Dec 1;244:118570. doi: 10.1016/j.neuroimage.2021.118570. Epub 2021 Sep 8. Neuroimage. 2021. PMID: 34508898 Review.
Cited by
-
C9orf72 gene networks in the human brain correlate with cortical thickness in C9-FTD and implicate vulnerable cell types.Front Neurosci. 2024 Feb 26;18:1258996. doi: 10.3389/fnins.2024.1258996. eCollection 2024. Front Neurosci. 2024. PMID: 38469573 Free PMC article.
-
The expression of insulin signaling and N-methyl-D-aspartate receptor genes in areas of gray matter atrophy is associated with cognitive function in type 2 diabetes.medRxiv [Preprint]. 2025 Apr 1:2025.03.26.25324696. doi: 10.1101/2025.03.26.25324696. medRxiv. 2025. Update in: J Alzheimers Dis. 2025 Aug 13:13872877251364906. doi: 10.1177/13872877251364906. PMID: 40236395 Free PMC article. Updated. Preprint.
-
Transcriptomic gradients of the human cerebellum.Imaging Neurosci (Camb). 2025 Feb 26;3:imag_a_00494. doi: 10.1162/imag_a_00494. eCollection 2025. Imaging Neurosci (Camb). 2025. PMID: 40800898 Free PMC article.
-
Beyond the usual suspects: multi-factorial computational models in the search for neurodegenerative disease mechanisms.Transl Psychiatry. 2024 Sep 23;14(1):386. doi: 10.1038/s41398-024-03073-w. Transl Psychiatry. 2024. PMID: 39313512 Free PMC article. Review.
-
New Challenges for Anatomists in the Era of Omics.Diagnostics (Basel). 2023 Sep 15;13(18):2963. doi: 10.3390/diagnostics13182963. Diagnostics (Basel). 2023. PMID: 37761332 Free PMC article. Review.
References
-
- Arnatkeviciute A., Fulcher B.D., Bellgrove M.A., Fornito A. Where the genome meets the connectome: Understanding how genes shape human brain connectivity. Neuroimage. 2021;244:118570. - PubMed
-
- Bigos K.L., Weinberger D.R. Imaging genetics—Days of future past. Neuroimage. 2010;53:804–809. - PubMed
-
- Fornito A., Arnatkevičiūtė A., Fulcher B.D. Bridging the gap between connectome and transcriptome. Trends Cogn Sci. 2019;23:34–50. - PubMed
-
- Thompson P.M., Cannon T.D., Narr K.L., van Erp T., Poutanen V.P., Huttunen M., et al. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

