Aberrant waste disposal in neurodegeneration: why improved sleep could be the solution
- PMID: 36324713
- PMCID: PMC9616228
- DOI: 10.1016/j.cccb.2021.100025
Aberrant waste disposal in neurodegeneration: why improved sleep could be the solution
Erratum in
-
Erratum regarding previously published articles.Cereb Circ Cogn Behav. 2022 Jan 26;3:100038. doi: 10.1016/j.cccb.2022.100038. eCollection 2022. Cereb Circ Cogn Behav. 2022. PMID: 36326279 Free PMC article.
Abstract
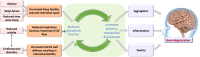
Sleep takes up a large percentage of our lives and the full functions of this state are still not understood. However, over the last 10 years a new and important function has emerged as a mediator of brain clearance. Removal of toxic metabolites and proteins from the brain parenchyma generated during waking activity and high levels of synaptic processing is critical to normal brain function and only enabled during deep sleep. Understanding of this process is revealing how impaired sleep contributes an important and likely causative role in the accumulation and aggregation of aberrant proteins such as β-amyloid and phosphorylated tau, as well as inflammation and neuronal damage. We are also beginning to understand how brain slow-wave activity interacts with vascular function allowing the flow of CSF and interstitial fluid to drain into the body's lymphatic system. New methodology is enabling visualization of this process in both animals and humans and is revealing how these processes break down during ageing and disease. With this understanding we can begin to envisage novel therapeutic approaches to the treatment of neurodegeneration, and how reversing sleep impairment in the correct manner may provide a way to slow these processes and improve brain function.
Keywords: AQP4, aquaporin-4; Alzheimer's disease; Amyloid; Aquaporin-4; Astrocyte; Aβ, beta amyloid; BOLD, blood-oxygen level dependent imaging; CAA, cerebral amyloid angiopathy; CSF, Cerebrospinal fluid; Clearance; EEG, electroencephalography; EMG, electromyography; Glymphatic; ISF, interstitial fluid; MCI, mild cognitive impairment; MRI, magnetic resonance imaging; NOS, nitric oxide synthase; NREM, non-rapid eye movement; OSA, obstructive sleep apnea; PET, positron emission tomography; REM, rapid-eye movement; SWA, slow wave activity; SWS, slow-wave sleep; Slow-wave sleep; iNPH, idiopathic normal pressure hydrocephalus.
Crown Copyright © 2021 Published by Elsevier B.V.
Figures
References
-
- Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R. Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener. 2016;11(1):74. doi: 10.1186/s13024-016-0138-8. Dec 8Erratum in: Mol Neurodegener. 2017 Jan 12;12 (1):3. PMID: 27931262; PMCID: PMC5146863. - DOI - PMC - PubMed
-
- Albargothy NJ, Johnston DA, MacGregor-Sharp M, Weller RO, Verma A, Hawkes CA, Carare RO. Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 2018;136(1):139–152. doi: 10.1007/s00401-018-1862-7. JulEpub 2018 May 12. PMID: 29754206; PMCID: PMC6015107. - DOI - PMC - PubMed
-
- Amara AW, Wood KH, Joop A, Memon RA, Pilkington J, Tuggle SC, Reams J, Barrett MJ, Edwards DA, Weltman AL, Hurt CP, Cutter G, Randomized Bamman MM. Controlled trial of exercise on objective and subjective sleep in Parkinson's disease. Mov. Disord. 2020;35(6):947–958. doi: 10.1002/mds.28009. JunEpub 2020 Feb 24. PMID: 32092190. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

