Right atrial appendage firing in atrial fibrillation
- PMID: 36324749
- PMCID: PMC9618623
- DOI: 10.3389/fcvm.2022.997998
Right atrial appendage firing in atrial fibrillation
Abstract
Background: The role of atrial fibrillation (AF) drivers located at the left atrium, superior vena cava, crista terminalis and coronary sinus (CS) is well established. While these regions are classically targeted during catheter ablation, the role of right atrial appendage (RAA) drivers has been incompletely investigated.
Objective: To determine the prevalence and electrophysiological characteristics of AF driver's arising from the RAA.
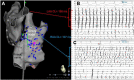
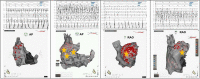
Materials and methods: We conducted a retrospective analysis of clinical and procedural data of 317 consecutive patients who underwent an AF ablation procedure after bi-atrial mapping (multipolar catheter). We selected patients who presented with a per-procedural RAA firing (RAAF). RAAF was defined as the recording of a sustained RAA EGM with a cycle length shorter than 120 ms or 120 < RAAF CL ≤ 130 ms and ratio RAA CL/CS CL ≤ 0.75.
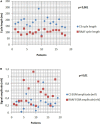
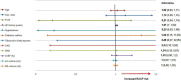
Results: Right atrial/atrium appendage firing was found in 22 patients. The prevalence was estimated at 7% (95% CI, 4-10). These patients were mostly men (72%), median age: 66 yo ± 8 without structural heart disease (77%). RAAFs were predominantly found in paroxysmal AF patients (63%, 32%, and 5% for paroxysmal, short standing and long-standing AF, respectively, p > 0.05). RAAF median cycle length was 117 ms ± 7 while CS cycle length was 180 ms ± 10 (p < 0.01).
Conclusion: In 317 consecutive AF ablation patients (22 patients, 7%) the presence of a high-voltage short-cycle-length right atrial appendage driver (RAAF) may conclusively be associated with AF termination. This case series exemplifies the not-so-uncommon role of the RAA in the perpetuation of AF.
Keywords: AF ablation; AF driver; dispersion; right atrial appendage; tailored ablation.
Copyright © 2022 Baptiste, Kalifa, Durand, Gitenay, Bremondy, Ayari, Maillot, Taormina, Fofana, Penaranda, Siame, Bars and Seitz.
Conflict of interest statement
Author EG received consultance fees from Abbott. Authors CB, JK, and JS received Speaker Fees from Biosense Webster, Abbott, Boston Scientific and are shareholders of VOLTA Medical. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, et al. Driver domains in persistent atrial fibrillation. Circulation. (2014) 130:530–8. - PubMed
-
- Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659–66. - PubMed
-
- Patterson E, Jackman WM, Beckman KJ, Lazzara R, Lockwood D, Scherlag BJ, et al. Spontaneous pulmonary vein firing in man: relationship to tachycardia-pause early afterdepolarizations and triggered arrhythmia in canine pulmonary veins in vitro. J Cardiovasc Electrophysiol. (2007) 18:1067–75. 10.1111/j.1540-8167.2007.00909.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

