This is a preprint.
Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters
- PMID: 36324798
- PMCID: PMC9628195
- DOI: 10.1101/2022.10.24.513619
Immunogenicity of the BA.5 Bivalent mRNA Vaccine Boosters
Update in
-
Immunogenicity of BA.5 Bivalent mRNA Vaccine Boosters.N Engl J Med. 2023 Feb 9;388(6):565-567. doi: 10.1056/NEJMc2213948. Epub 2023 Jan 11. N Engl J Med. 2023. PMID: 36630611 Free PMC article. No abstract available.
Abstract
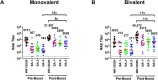
Waning immunity following mRNA vaccination and the emergence of SARS-CoV-2 variants has led to reduced mRNA vaccine efficacy against both symptomatic infection and severe disease. Bivalent mRNA boosters expressing the Omicron BA.5 and ancestral WA1/2020 Spike proteins have been developed and approved, because BA.5 is currently the dominant SARS-CoV-2 variant and substantially evades neutralizing antibodies (NAbs). Our data show that BA.5 NAb titers were comparable following monovalent and bivalent mRNA boosters.
Conflict of interest statement
Conflicts of Interest
The authors report no conflicts of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
