Modulation of thromboinflammation in hospitalized COVID-19 patients with aprotinin, low molecular weight heparin, and anakinra: The DAWn-Antico study
- PMID: 36324831
- PMCID: PMC9618401
- DOI: 10.1002/rth2.12826
Modulation of thromboinflammation in hospitalized COVID-19 patients with aprotinin, low molecular weight heparin, and anakinra: The DAWn-Antico study
Abstract
Background: Thromboinflammation plays a central role in severe COVID-19. The kallikrein pathway activates both inflammatory pathways and contact-mediated coagulation. We investigated if modulation of the thromboinflammatory response improves outcomes in hospitalized COVID-19 patients.
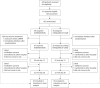
Methods: In this multicenter open-label randomized clinical trial (EudraCT 2020-001739-28), patients hospitalized with COVID-19 were 1:2 randomized to receive standard of care (SOC) or SOC plus study intervention. The intervention consisted of aprotinin (2,000,000 IE IV four times daily) combined with low molecular weight heparin (LMWH; SC 50 IU/kg twice daily on the ward, 75 IU/kg twice daily in intensive care). Additionally, patients with predefined hyperinflammation received the interleukin-1 receptor antagonist anakinra (100 mg IV four times daily). The primary outcome was time to a sustained 2-point improvement on the 7-point World Health Organization ordinal scale for clinical status, or discharge.
Findings: Between 24 June 2020 and 1 February 2021, 105 patients were randomized, and 102 patients were included in the full analysis set (intervention N = 67 vs. SOC N = 35). Twenty-five patients from the intervention group (37%) received anakinra. The intervention did not affect the primary outcome (HR 0.77 [CI 0.50-1.19], p = 0.24) or mortality (intervention n = 3 [4.6%] vs. SOC n = 2 [5.7%], HR 0.82 [CI 0.14-4.94], p = 0.83). There was one treatment-related adverse event in the intervention group (hematuria, 1.49%). There was one thrombotic event in the intervention group (1.49%) and one in the SOC group (2.86%), but no major bleeding.
Conclusions: In hospitalized COVID-19 patients, modulation of thromboinflammation with high-dose aprotinin and LMWH with or without anakinra did not improve outcome in patients with moderate to severe COVID-19.
Keywords: COVID‐19; anakinra; aprotinin; heparin; inflammation; low‐molecular‐weight; thrombosis.
© 2022 The Authors. Research and Practice in Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis (ISTH).
Figures

References
LinkOut - more resources
Full Text Sources

