Genetic profiling of human bone marrow mesenchymal stromal cells after in vitro expansion in clinical grade human platelet lysate
- PMID: 36324892
- PMCID: PMC9621119
- DOI: 10.3389/fbioe.2022.1008271
Genetic profiling of human bone marrow mesenchymal stromal cells after in vitro expansion in clinical grade human platelet lysate
Abstract
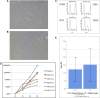
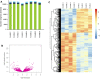
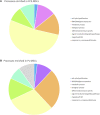
Mesenchymal stromal cells (MSCs) are non-hematopoietic cells that have a broad therapeutic potential. To obtain sufficient cells for clinical application, they must be expanded ex vivo. In the initial expansion protocols described, fetal calf serum (FCS) was used as the reference growth supplement, but more recently different groups started to replace FCS with platelet lysate (PL). We investigated in this study the impact of the culture supplement on gene expression of MSCs. Human bone marrow derived MSCs were expanded in vitro in FCS and PL supplemented medium. We found that MSCs expanded in PL-containing medium (PL-MSCs) express typical MSC immunomorphological features and can migrate, as their counterparts expanded in FCS-containing medium, through a layer of endothelial cells in vitro. Additionally, they show an increased proliferation rate compared to MSCs expanded in FCS medium (FCS-MSCs). RNA sequencing performed for MSCs cultured in both types of expansion medium revealed a large impact of the choice of growth supplement on gene expression: 1974 genes were at least twofold up- or downregulated. We focused on impact of genes involved in apoptosis and senescence. Our data showed that PL-MSCs express more anti-apoptotic genes and FCS-MSCs more pro-apoptotic genes. FCS-MSCs showed upregulation of senescence-related genes after four passages whereas this was rarer in PL-MSCs at the same timepoint. Since PL-MSCs show higher proliferation rates and anti-apoptotic gene expression, they might acquire features that predispose them to malignant transformation. We screened 10 MSC samples expanded in PL-based medium for the presence of tumor-associated genetic variants using a 165 gene panel and detected only 21 different genetic variants. According to our analysis, none of these were established pathogenic mutations. Our data show that differences in culture conditions such as growth supplement have a significant impact on the gene expression profile of MSCs and favor the use of PL over FCS for expansion of MSCs.
Keywords: expansion; gene expression; mesenchymal stromal cell; platelet lysate; transformation.
Copyright © 2022 De Becker, Heestermans, De Brouwer, Bockstaele, Maes and Van Riet.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

