Myoclonus and other jerky movement disorders
- PMID: 36324989
- PMCID: PMC9619152
- DOI: 10.1016/j.cnp.2022.09.003
Myoclonus and other jerky movement disorders
Abstract
Myoclonus and other jerky movements form a large heterogeneous group of disorders. Clinical neurophysiology studies can have an important contribution to support diagnosis but also to gain insight in the pathophysiology of different kind of jerks. This review focuses on myoclonus, tics, startle disorders, restless legs syndrome, and periodic leg movements during sleep. Myoclonus is defined as brief, shock-like movements, and subtypes can be classified based the anatomical origin. Both the clinical phenotype and the neurophysiological tests support this classification: cortical, cortical-subcortical, subcortical/non-segmental, segmental, peripheral, and functional jerks. The most important techniques used are polymyography and the combination of electromyography-electroencephalography focused on jerk-locked back-averaging, cortico-muscular coherence, and the Bereitschaftspotential. Clinically, the differential diagnosis of myoclonus includes tics, and this diagnosis is mainly based on the history with premonitory urges and the ability to suppress the tic. Electrophysiological tests are mainly applied in a research setting and include the Bereitschaftspotential, local field potentials, transcranial magnetic stimulation, and pre-pulse inhibition. Jerks due to a startling stimulus form the group of startle syndromes. This group includes disorders with an exaggerated startle reflex, such as hyperekplexia and stiff person syndrome, but also neuropsychiatric and stimulus-induced disorders. For these disorders polymyography combined with a startling stimulus can be useful to determine the pattern of muscle activation and thus the diagnosis. Assessment of symptoms in restless legs syndrome and periodic leg movements during sleep can be performed with different validated scoring criteria with the help of electromyography.
Keywords: Deep Brain Stimulation; EEG; EMG; Local field potentials; Myoclonus; Neurophysiology; PLMS; RLS; Startle; Tics; Tourette disorder; Transcranial Magnetic Stimulation.
© 2022 International Federation of Clinical Neurophysiology. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
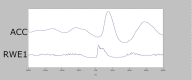
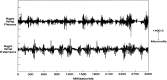
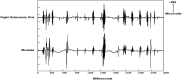
Figures












References
-
- Allen R.P., Picchietti D., Hening W.A., Trenkwalder C., Walters A.S., Montplaisi J., et al. Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–119. doi: 10.1016/S1389-9457(03)00010-8. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

