Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes
- PMID: 36325344
- PMCID: PMC9619036
- DOI: 10.3389/fimmu.2022.930511
Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes
Abstract
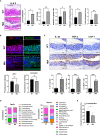
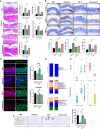
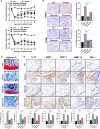
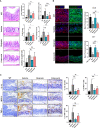
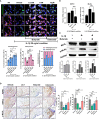
Osteoarthritis (OA) reduces the quality of life as a result of the pain caused by continuous joint destruction. Inactivated Lactobacillus (LA-1) ameliorated osteoarthritis and protected cartilage by modulating inflammation. In this study, we evaluated the mechanism by which live LA-1 ameliorated OA. To investigate the effect of live LA-1 on OA progression, we administered LA-1 into monosodium iodoacetate (MIA)-induced OA animals. The pain threshold, cartilage damage, and inflammation of the joint synovial membrane were improved by live LA-1. Furthermore, the analysis of intestinal tissues and feces in the disease model has been shown to affect the systems of the intestinal system and improve the microbiome environment. Interestingly, inflammation of the intestinal tissue was reduced, and the intestinal microbiome was altered by live LA-1. Live LA-1 administration led to an increase in the level of Faecalibacterium which is a short-chain fatty acid (SCFA) butyrate-producing bacteria. The daily supply of butyrate, a bacterial SCFA, showed a tendency to decrease necroptosis, a type of abnormal cell death, by inducing autophagy and reversing impaired autophagy by the inflammatory environment. These results suggest that OA is modulated by changes in the gut microbiome, suggesting that activation of autophagy can reduce aberrant cell death. In summary, live LA-1 or butyrate ameliorates OA progression by modulating the gut environment and autophagic flux. Our findings suggest the regulation of the gut microenvironment as a therapeutic target for OA.
Keywords: LA-1; butyrate; inflammation; microbiota; necroptosis; osteoarthritis.
Copyright © 2022 Cho, Na, Jhun, Woo, Lee, Lee, Lee, Um, Kim, Park and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Briggs AM, Cross MJ, Hoy DG, Sanchez-Riera L, Blyth FM, Woolf AD, et al. . Musculoskeletal health conditions represent a global threat to healthy aging: A report for the 2015 world health organization world report on ageing and health. Gerontologist (2016) 56 Suppl 2:S243–55. doi: 10.1093/geront/gnw002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

