Interspecies relationships between nosocomial pathogens associated to preterm infants and lactic acid bacteria in dual-species biofilms
- PMID: 36325465
- PMCID: PMC9618709
- DOI: 10.3389/fcimb.2022.1038253
Interspecies relationships between nosocomial pathogens associated to preterm infants and lactic acid bacteria in dual-species biofilms
Abstract
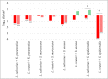
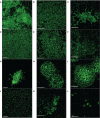
The nasogastric enteral feeding tubes (NEFTs) used to feed preterm infants are commonly colonized by bacteria with the ability to form complex biofilms in their inner surfaces. Among them, staphylococci (mainly Staphylococcus epidermidis and Staphylococcus aureus) and some species belonging to the Family Enterobacteriaceae are of special concern since they can cause nosocomial infections in this population. NETF-associated biofilms can also include lactic acid bacteria (LAB), with the ability to compete with pathogenic species for nutrients and space. Ecological interactions among the main colonizers of these devices have not been explored yet; however, such approach could guide future strategies involving the pre-coating of the inner surfaces of NEFTs with well adapted LAB strains in order to reduce the rates of nosocomial infections in neonatal intensive care units (NICUs). In this context, this work implied the formation of dual-species biofilms involving one LAB strain (either Ligilactobacillus salivarius 20SNG2 or Limosilactobacillus reuteri 7SNG3) and one nosocomial strain (either Klebsiella pneumoniae 9SNG3, Serratia marcescens 10SNG3, Staphylococcus aureus 45SNG3 or Staphylococcus epidermidis 46SNG3). The six strains used in this study had been isolated from the inner surface of NEFTs. Changes in adhesion ability of the pathogens were characterized using a culturomic approach. Species interactions and structural changes of the resulting biofilms were analyzed using scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). No aggregation was observed in dual-species biofilms between any of the two LAB strains and either K. pneumoniae 9SNG3 or S. marcescens 10SNG3. In addition, biofilm thickness and volume were reduced, suggesting that both LAB strains can control the capacity to form biofilms of these enterobacteria. In contrast, a positive ecological relationship was observed in the combination L. reuteri 7SNG3-S. aureus 45SNG3. This relationship was accompanied by a stimulation of S. aureus matrix production when compared with its respective monospecies biofilm. The knowledge provided by this study may guide the selection of potentially probiotic strains that share the same niche with nosocomial pathogens, enabling the establishment of a healthier microbial community inside NEFTs.
Keywords: Serratia marcescens; Staphylococcus aureus; Staphylococcus epidermidis; biofilms; klebsiella pneumoniae; lactic acid bacteria; nasogastric enteral feeding tubes; preterm infants.
Copyright © 2022 Jara, Jurado, Almendro-Vedia, López-Montero, Fernández, Rodríguez and Orgaz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer RC declared a past co-authorship with the author JR to the handling editor.
Figures




References
-
- Ahmed A., Dachang W., Lei Z., Jianjun L., Juanjuan Q., Yi X. (2014). Effect of Lactobacillus species on Streptococcus mutans biofilm formation. Pak. J. Pharm. Sci. 27, 1–6. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

