A mechanistic study of the dopant-induced breakdown in halide perovskites using solid state energy storage devices
- PMID: 36325485
- PMCID: PMC9555316
- DOI: 10.1039/d2ee01754g
A mechanistic study of the dopant-induced breakdown in halide perovskites using solid state energy storage devices
Abstract
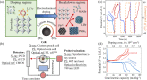
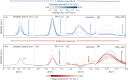
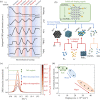
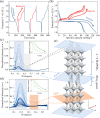
Doping halide perovskites (HPs) with extrinsic species, such as alkali metal ions, plays a critical, albeit often elusive role in optimising optoelectronic devices. Here, we use solid state lithium ion battery inspired devices with a polyethylene oxide-based polymer electrolyte to dope HPs controllably with lithium ions. We perform a suite of operando material analysis techniques while dynamically varying Li doping concentrations. We determine and quantify three doping regimes; a safe regime, with doping concentrations of <1020 cm-3 (2% Li : Pb mol%) in which the HP may be modified without detrimental effect to its structure; a minor decomposition regime, in which the HP is partially transformed but remains the dominant species; and a major decomposition regime in which the perovskite is superseded by new phases. We provide a mechanistic description of the processes mediating between each stage and find evidence for metallic Pb(0), LiBr and LiPbBr2 as final decomposition products. Combining results from synchrotron X-ray diffraction measurements with in situ photoluminescence and optical reflection microscopy studies, we distinguish the influences of free charge carriers and intercalated lithium independently. We find that the charge density is equally as important as the geometric considerations of the dopant species and thereby provide a quantitative framework upon which the future design of doped-perovskite energy devices should be based.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
References
-
- Park N.-G. Perovskite solar cells: an emerging photovoltaic technology. Mater. Today. 2015;18:65–72. doi: 10.1016/j.mattod.2014.07.007. - DOI
-
- Snaith H. J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013;4:3623–3630. doi: 10.1021/jz4020162. - DOI
-
- Green M. Dunlop E. Hohl-Ebinger J. Yoshita M. Kopidakis N. Hao X. Solar cell efficiency tables (version 59) Prog. Photovoltaics. 2022;29:3–15.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
