A novel thymidine phosphorylase to synthesize (halogenated) anticancer and antiviral nucleoside drugs in continuous flow
- PMID: 36325519
- PMCID: PMC9575728
- DOI: 10.1039/d2cy00751g
A novel thymidine phosphorylase to synthesize (halogenated) anticancer and antiviral nucleoside drugs in continuous flow
Abstract
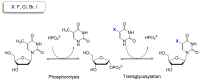
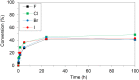
Four pharmaceutically relevant nucleoside analogues (5-fluoro-2'-deoxyuridine, 5-chloro-2'-deoxyuridine, 5-bromo-2'-deoxyuridine, and 5-iodo-2'-deoxyuridine) have been synthesized by using a novel thymidine phosphorylase from the halotolerant H. elongata (HeTP). Following enzyme immobilization on microbeads, the biocatalyst was implemented as a packed-bed reactor for the continuous production of halogenated nucleosides, achieving up to 90% conversion at the 10 mM scale with 30 min residence time. Taking the synthesis of floxuridine (5-fluoro-2'-deoxyuridine) as a study case, we obtained the highest space-time yield (5.5 g L-1 h-1) reported to date. In addition, bioinformatic tools such as MD analysis and CapiPy have contributed to shine light on the catalytic performance of HeTP as well as its immobilization, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
Similar articles
-
Sustainable Flow-Synthesis of (Bulky) Nucleoside Drugs by a Novel and Highly Stable Nucleoside Phosphorylase Immobilized on Reusable Supports.ChemSusChem. 2022 Jan 10;15(1):e202102030. doi: 10.1002/cssc.202102030. Epub 2021 Nov 27. ChemSusChem. 2022. PMID: 34726353 Free PMC article.
-
Immobilized enzyme reactors based on nucleoside phosphorylases and 2'-deoxyribosyltransferase for the in-flow synthesis of pharmaceutically relevant nucleoside analogues.Bioresour Technol. 2020 Jul;307:123258. doi: 10.1016/j.biortech.2020.123258. Epub 2020 Mar 26. Bioresour Technol. 2020. PMID: 32247276
-
Production of Modified Nucleosides in a Continuous Enzyme Membrane Reactor.Int J Mol Sci. 2023 Mar 23;24(7):6081. doi: 10.3390/ijms24076081. Int J Mol Sci. 2023. PMID: 37047056 Free PMC article.
-
Acquisition of human concentrative nucleoside transporter 2 (hcnt2) activity by gene transfer confers sensitivity to fluoropyrimidine nucleosides in drug-resistant leukemia cells.Mol Pharmacol. 2001 Nov;60(5):1143-52. doi: 10.1124/mol.60.5.1143. Mol Pharmacol. 2001. PMID: 11641443
-
Bicyclic pyrimidine nucleoside analogues (BCNAs) as highly selective and potent inhibitors of varicella-zoster virus replication.J Antimicrob Chemother. 2002 Jul;50(1):5-9. doi: 10.1093/jac/dkf037. J Antimicrob Chemother. 2002. PMID: 12096000 Review.
Cited by
-
Gram-scale enzymatic synthesis of 2'-deoxyribonucleoside analogues using nucleoside transglycosylase-2.Chem Sci. 2024 Aug 27;15(37):15399-407. doi: 10.1039/d4sc04938a. Online ahead of print. Chem Sci. 2024. PMID: 39234214 Free PMC article.
-
Light-Driven Photobiocatalytic Oxyfunctionalization in a Continuous Reactor System without External Oxygen Supply.ACS Sustain Chem Eng. 2025 Mar 5;13(10):3939-3950. doi: 10.1021/acssuschemeng.4c08560. eCollection 2025 Mar 17. ACS Sustain Chem Eng. 2025. PMID: 40115393 Free PMC article.
-
Halomonas elongata: a microbial source of highly stable enzymes for applied biotechnology.Appl Microbiol Biotechnol. 2023 May;107(10):3183-3190. doi: 10.1007/s00253-023-12510-7. Epub 2023 Apr 13. Appl Microbiol Biotechnol. 2023. PMID: 37052635 Free PMC article. Review.
-
Nucleoside Analogs: A Review of Its Source and Separation Processes.Molecules. 2023 Oct 12;28(20):7043. doi: 10.3390/molecules28207043. Molecules. 2023. PMID: 37894522 Free PMC article. Review.
References
-
- Galmarini C. M. Electron. J. Oncol. 2002;3:22–32.
-
- HS/CN8 classification reference list for dataset ‘EU trade since 2015 of COVID-19 medical supplies’, https://ec.europa.eu/eurostat/documents/6842948/11003521/Corona+related+..., (accessed 11 April 2022)
LinkOut - more resources
Full Text Sources
