Celecoxib impairs primary human myoblast proliferation and differentiation independent of cyclooxygenase 2 inhibition
- PMID: 36325583
- PMCID: PMC9630763
- DOI: 10.14814/phy2.15481
Celecoxib impairs primary human myoblast proliferation and differentiation independent of cyclooxygenase 2 inhibition
Abstract
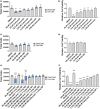
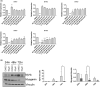
The use of non-steroidal anti-inflammatory drugs (NSAIDs) for treatment of musculoskeletal injuries is commonplace in the general, athletic, and military populations. While NSAIDs have been studied in a variety of tissues, the effects of NSAIDs on skeletal muscle have not been fully defined. To address this, we investigated the degree to which the cyclooxygenase (COX)-2-selective NSAID celecoxib affects muscle cell proliferation, differentiation, anabolic signaling, and mitochondrial function in primary human skeletal myoblasts and myotubes. Primary muscle cells were treated with celecoxib or NS-398 (a pharmacological inhibitor of COX-2) as a control. Celecoxib administration significantly reduced myoblast proliferation, viability, fusion, and myotube area in a dose-dependent manner, whereas NS-398 had no effect on any of these outcomes. Celecoxib treatment was also associated with reduced phosphorylation of ribosomal protein S6 in myoblasts, and reduced phosphorylation of AKT, p70S6K, S6, and ERK in myotubes. In contrast, NS-398 did not alter phosphorylation of these molecules in myoblasts or myotubes. In myoblasts, celecoxib significantly reduced mitochondrial membrane potential and respiration, as evidenced by the decreased citric acid cycle (CAC) intermediates cis-aconitic acid, alpha-keto-glutarate acid, succinate acid, and malic acid. Similar results were observed in myotubes, although celecoxib also reduced pyruvic acid, citric acid, and fumaric acid. NS-398 did not affect CAC intermediates in myoblasts or myotubes. Together, these data reveal that celecoxib inhibits proliferation, differentiation, intracellular signaling, and mitochondrial function in primary human myoblasts and myotubes independent of its function as a COX-2 inhibitor.
Keywords: celecoxib; cyclooxygenase; inflammation; mitochondria; myoblast; non-steroidal anti-inflammatory drug (NSAID).
Published 2022. This article is a U.S. Government work and is in the public domain in the USA. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The authors have no conflict of interests to report.
Figures





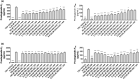




References
-
- Bondesen, B. A. , Mills, S. T. , Kegley, K. M. , & Pavlath, G. K. (2004). The COX‐2 pathway is essential during early stages of skeletal muscle regeneration. American Journal of Physiology. Cell Physiology, 287(2), C475–C483. - PubMed
-
- Bondesen, B. A. , Mills, S. T. , & Pavlath, G. K. (2006). The COX‐2 pathway regulates growth of atrophied muscle via multiple mechanisms. American Journal of Physiology. Cell Physiology, 290(6), C1651–C1659. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

