Dynamic cerebral autoregulation is intact in chronic kidney disease
- PMID: 36325592
- PMCID: PMC9630754
- DOI: 10.14814/phy2.15495
Dynamic cerebral autoregulation is intact in chronic kidney disease
Abstract
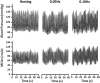
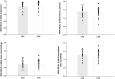
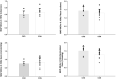
Chronic Kidney Disease (CKD) patients experience an elevated risk for cerebrovascular disease. One factor that may contribute to this heightened risk is an impairment in dynamic cerebral autoregulation, the mechanism by which cerebral vessels modulate cerebral blood flow during fluctuations in arterial pressure. We hypothesized that dynamic cerebral autoregulation would be impaired in CKD. To test this hypothesis, we compared dynamic cerebral autoregulation between CKD patients stages III-IV and matched controls (CON) without CKD. Fifteen patients with CKD and 20 CON participants performed 2, 5-minute bouts of repeated sit-to-stand maneuvers at 0.05 Hz and 0.10 Hz while mean arterial pressure (MAP, via finger photoplethysmography) and middle cerebral artery blood velocity (MCAv, via transcranial Doppler ultrasound) were measured continuously. Cerebral autoregulation was characterized by performing a transfer function analysis (TFA) on the MAP-MCAv relationship to derive coherence, phase, gain, and normalized gain (nGain). We observed no group differences in any of the TFA metrics during the repeated sit-to-stand maneuvers. During the 0.05 Hz maneuver, Coherence: CKD = 0.83 ± 0.13, CON = 0.85 ± 0.12, Phase (radians): CKD = 1.39 ± 0.41, CON = 1.25 ± 0.30, Gain (cm/s/mmHg): CKD = 0.69 ± 0.20, CON = 0.71 ± 0.22, nGain (%/mmHg): CKD = 1.26 ± 0.35, CON = 1.20 ± 0.28, p ≥ 0.24. During the 0.10 Hz maneuver (N = 6 CKD and N = 12 CON), Coherence: CKD = 0.61 ± 0.10, CON = 0.67 ± 0.11, Phase (radians): CKD = 1.43 ± 0.26, CON = 1.30 ± 0.23, Gain (cm/s/mmHg): CKD = 0.75 ± 0.15, CON = 0.84 ± 0.26, nGain (%/mmHg): CKD = 1.50 ± 0.28, CON = 1.29 ± 0.24, p ≥ 0.12. Contrary to our hypothesis, dynamic cerebral autoregulation remains intact in CKD stages III-IV. These findings suggest that other mechanisms likely contribute to the increased cerebrovascular disease burden experienced by this population. Future work should determine if other cerebrovascular regulatory mechanisms are impaired and related to cerebrovascular disease risk in CKD.
Keywords: cerebral blood flow; cerebrovascular disease; renal disease; transfer function analysis.
© 2022 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Figures

References
-
- Aries, M. J. , Elting, J. W. , De Keyser, J. , Kremer, B. P. , & Vroomen, P. C. (2010). Cerebral autoregulation in stroke: A review of transcranial Doppler studies. Stroke, 41, 2697–2704. - PubMed
-
- Chi, N. F. , Hu, H. H. , Wang, C. Y. , Chan, L. , Peng, C. K. , Novak, V. , & Hu, C. J. (2018). Dynamic cerebral autoregulation is an independent functional outcome predictor of mild acute ischemic stroke. Stroke, 49, 2605–2611. - PubMed
-
- Claassen, J. A. , Meel‐van den Abeelen, A. S. , Simpson, D. M. , Panerai, R. B. , & international Cerebral Autoregulation Research N . (2016). Transfer function analysis of dynamic cerebral autoregulation: A white paper from the International Cerebral Autoregulation Research Network. Journal of Cerebral Blood Flow and Metabolism, 36, 665–680. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

