The cooperative folding of annexin A2 relies on a transient nonnative intermediate
- PMID: 36325614
- PMCID: PMC9748365
- DOI: 10.1016/j.bpj.2022.10.043
The cooperative folding of annexin A2 relies on a transient nonnative intermediate
Abstract
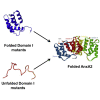
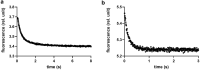
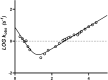
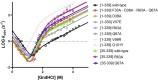
Annexins (Anxs) are a family of highly homologous proteins that bind and aggregate lipid vesicles in the presence of calcium. All members of the family contain a variable N-terminus determining specific functions, followed by a conserved core region responsible for the general calcium-dependent lipid-binding property. The core structure consists of four homologous domains (DI-DIV), each consisting of a right-handed super-helix of five α-helices. We present data from a combination of site-directed mutagenesis, NMR, and circular dichroism showing that the G25-D34 region of the N-terminus as well as the contacts between residues D38A, R63A, and Q67A of AnxA2-DI are crucial for the autonomous folding and stability of DI of AnxA2. However, we also show that the folding of the full-length protein is very robust in that mutations and truncations that disrupted the folding of AnxA2-DI did not abolish the folding of full-length AnxA2, only lowering its thermal stability. This robustness of the folding of full-length AnxA2 is likely to be mediated by the existence of at least one transient nonnative intermediate as suggested by our kinetic data using stopped-flow fluorescence experiments. We also show that hydrophobic amino acids in AnxA2-DI involved in interfacial contacts with AnxA2-DIV are important for the cooperative folding and stability of the full-length protein. Mutating all of the V57E-V98R-G101Y residues in AnxA2-DI did not affect the folding of the domain, only its stability, but prevented the cooperative folding of the full-length protein. Our collective results favor a highly cooperative and robust folding process mediated by alternative intermediate steps. Since AnxA2 is a multifunctional protein involved in several steps of the progression of cell transformation, these data on structure and folding pathways are therefore crucial to designing anticancer drugs targeting AnxA2.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
The mRNA-binding site of annexin A2 resides in helices C-D of its domain IV.J Mol Biol. 2007 May 18;368(5):1367-78. doi: 10.1016/j.jmb.2007.02.094. Epub 2007 Mar 7. J Mol Biol. 2007. PMID: 17395201
-
Lipid segregation and membrane budding induced by the peripheral membrane binding protein annexin A2.J Biol Chem. 2013 Aug 23;288(34):24764-76. doi: 10.1074/jbc.M113.474023. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861394 Free PMC article.
-
Bridging of membrane surfaces by annexin A2.Sci Rep. 2018 Oct 2;8(1):14662. doi: 10.1038/s41598-018-33044-3. Sci Rep. 2018. PMID: 30279443 Free PMC article.
-
Multiple roles of annexin A2 in post-transcriptional regulation of gene expression.Curr Protein Pept Sci. 2012 Jun;13(4):401-12. doi: 10.2174/138920312801619402. Curr Protein Pept Sci. 2012. PMID: 22708494 Review.
-
Protein phosphorylation and its role in the regulation of Annexin A2 function.Biochim Biophys Acta Gen Subj. 2017 Nov;1861(11 Pt A):2515-2529. doi: 10.1016/j.bbagen.2017.08.024. Epub 2017 Sep 1. Biochim Biophys Acta Gen Subj. 2017. PMID: 28867585 Review.
References
-
- Gerke V., Moss S.E. Annexins: from structure to function. Physiol. Rev. 2002;82:331–371. - PubMed
-
- Gerke V., Creutz C.E., Moss S.E. Annexins: linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. - PubMed
-
- Rescher U., Gerke V. Annexins--unique membrane binding proteins with diverse functions. J. Cell Sci. 2004;117:2631–2639. - PubMed
-
- Vedeler A., Hollås H., et al. Raddum A.M. Multiple roles of annexin A2 in post-transcriptional regulation of gene expression. Curr. Protein Pept. Sci. 2012;13:401–412. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

