First-in-human study of deucravacitinib: A selective, potent, allosteric small-molecule inhibitor of tyrosine kinase 2
- PMID: 36325947
- PMCID: PMC9841305
- DOI: 10.1111/cts.13435
First-in-human study of deucravacitinib: A selective, potent, allosteric small-molecule inhibitor of tyrosine kinase 2
Abstract
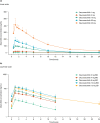
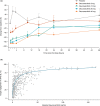
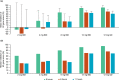
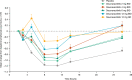
This randomized, double-blind, single- and multiple-ascending dose study assessed the pharmacokinetics (PKs), pharmacodynamics, and safety of deucravacitinib (Sotyktu™), a selective and potent small-molecule inhibitor of tyrosine kinase 2, in 100 (75 active, 25 placebo) healthy volunteers (NCT02534636). Deucravacitinib was rapidly absorbed, with a half-life of 8-15 h, and 1.4-1.9-fold accumulation after multiple dosing. Deucravacitinib inhibited interleukin (IL)-12/IL-18-induced interferon (IFN)γ production ex vivo in a dose- and concentration-dependent manner. Following in vivo challenge with IFNα-2a, deucravacitinib demonstrated dose-dependent inhibition of lymphocyte count decreases and expression of 53 IFN-regulated genes. There were no serious adverse events (AEs); the overall frequency of AEs was similar in the deucravacitinib (64%) and placebo (68%) groups. In this first-in-human study, deucravacitinib inhibited IL-12/IL-23 and type I IFN pathways in healthy volunteers, with favorable PK and safety profiles. Deucravacitinib is a promising therapeutic option for immune-mediated diseases, including Crohn's disease, psoriasis, psoriatic arthritis, and systemic lupus erythematosus.
© 2022 Bristol Myers Squibb. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
I.M.C., U.A., I.G.G., and B.M. are employees of Bristol Myers Squibb and receive salaries and stock commensurate with employment. L.H., Y.L., and D.B. were employees of Bristol Myers Squibb at the time this research was conducted.
Figures
References
-
- Bristol Myers Squibb . SOTYKTU (deucravacitinib) prescribing information; 2022. Accessed May 10, 2022. https://packageinserts.bms.com/pi/pi_sotyktu.pdf
-
- Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL‐12 and IL‐23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139‐156. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

