Developing a transwell millifluidic device for studying blood-brain barrier endothelium
- PMID: 36326069
- PMCID: PMC11416711
- DOI: 10.1039/d2lc00657j
Developing a transwell millifluidic device for studying blood-brain barrier endothelium
Abstract
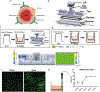
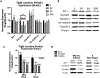
Blood-brain barrier (BBB) endothelial cell (EC) function depends on flow conditions and on supportive cells, like pericytes and astrocytes, which have been shown to be both beneficial and detrimental for brain EC function. Most studies investigating BBB EC function lack physiological relevance, using sub-physiological shear stress magnitudes and/or omitting pericytes and astrocytes. In this study, we developed a millifluidic device compatible with standard transwell inserts to investigate BBB function. In contrast to standard polydimethylsiloxane (PDMS) microfluidic devices, this model allows for easy, reproducible shear stress exposure without common limitations of PDMS devices such as inadequate nutrient diffusion and air bubble formation. In no-flow conditions, we first used the device to examine the impact of primary human pericytes and astrocytes on human brain microvascular EC (HBMEC) barrier integrity. Astrocytes, pericytes, and a 1-to-1 ratio of both cell types increased HBMEC barrier integrity via reduced 3 and 40 kDa fluorescent dextran permeability and increased claudin-5 expression. There were differing levels of low 3 kDa permeability in HBMEC-pericyte, HBMEC-astrocyte, and HBMEC-astrocyte-pericyte co-cultures, while levels of low 40 kDa permeability were consistent across co-cultures. The 3 kDa findings suggest that pericytes provide more barrier support to the BBB model compared to astrocytes, although both supportive cell types are permeability reducers. Incorporation of 24-hour 12 dynes per cm2 flow significantly reduced dextran permeability in HBMEC monolayers, but not in the tri-culture model. These results indicate that tri-culture may exert more pronounced impact on overall BBB permeability than flow exposure. In both cases, monolayer and tri-culture, flow exposure interestingly reduced HBMEC expression of both claudin-5 and occludin. ZO-1 expression, and localization at cell-cell junctions increased in the tri-culture but exhibited no apparent change in the HBMEC monolayer. Under flow conditions, we also observed HBMEC alignment in the tri-culture but not in HBMEC monolayers, indicating supportive cells and flow are both essential to observe brain EC alignment in vitro. Collectively, these results support the necessity of physiologically relevant, multicellular BBB models when investigating BBB EC function. Consideration of the roles of shear stress and supportive cells within the BBB is critical for elucidating the physiology of the neurovascular unit.
Conflict of interest statement
Conflicts of interest
The authors declare that U.S. Patent Application (No. 17/454768) has been filed for Hanging Cell Culture Millifluidic Device.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

