Observation of Cation Chromophore Photoisomerization of a Fluorescent Protein Using Millisecond Synchrotron Serial Crystallography and Infrared Vibrational and Visible Spectroscopy
- PMID: 36326150
- PMCID: PMC9677427
- DOI: 10.1021/acs.jpcb.2c06780
Observation of Cation Chromophore Photoisomerization of a Fluorescent Protein Using Millisecond Synchrotron Serial Crystallography and Infrared Vibrational and Visible Spectroscopy
Abstract
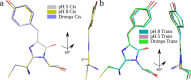
The chromophores of reversibly switchable fluorescent proteins (rsFPs) undergo photoisomerization of both the trans and cis forms. Concurrent with cis/trans photoisomerisation, rsFPs typically become protonated on the phenolic oxygen resulting in a blue shift of the absorption. A synthetic rsFP referred to as rsEospa, derived from EosFP family, displays the same spectroscopic behavior as the GFP-like rsFP Dronpa at pH 8.4 and involves the photoconversion between nonfluorescent neutral and fluorescent anionic chromophore states. Millisecond time-resolved synchrotron serial crystallography of rsEospa at pH 8.4 shows that photoisomerization is accompanied by rearrangements of the same three residues as seen in Dronpa. However, at pH 5.5 we observe that the OFF state is identified as the cationic chromophore with additional protonation of the imidazolinone nitrogen which is concurrent with a newly formed hydrogen bond with the Glu212 carboxylate side chain. FTIR spectroscopy resolves the characteristic up-shifted carbonyl stretching frequency at 1713 cm-1 for the cationic species. Electronic spectroscopy furthermore distinguishes the cationic absorption band at 397 nm from the neutral species at pH 8.4 seen at 387 nm. The observation of photoisomerization of the cationic chromophore state demonstrates the conical intersection for the electronic configuration, where previously fluorescence was proposed to be the main decay route for states containing imidazolinone nitrogen protonation. We present the full time-resolved room-temperature X-ray crystallographic, FTIR, and UV/vis assignment and photoconversion modeling of rsEospa.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Pletneva N. V.; Pletnev V. Z.; Shemiakina I. I.; Chudakov D. M.; Artemyev I.; Wlodawer A.; Dauter Z.; Pletnev S. Crystallographic Study of Red Fluorescent Protein EqFP578 and Its Far-Red Variant Katushka Reveals Opposite Ph-Induced Isomerization of Chromophore. Protein Sci. 2011, 20 (7), 1265–1274. 10.1002/pro.654. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

