Critical considerations of the contribution of the corticomotoneuronal pathway to central fatigue
- PMID: 36326193
- PMCID: PMC9772161
- DOI: 10.1113/JP282564
Critical considerations of the contribution of the corticomotoneuronal pathway to central fatigue
Abstract
Neural drive originating in higher brain areas reaches exercising limb muscles through the corticospinal-motoneuronal pathway, which links the motor cortex and spinal motoneurones. The properties of this pathway have frequently been observed to change during fatiguing exercise in ways that could influence the development of central fatigue (i.e. the progressive reduction in voluntary muscle activation). However, based on differences in motor cortical and motoneuronal excitability between exercise modalities (e.g. single-joint vs. locomotor exercise), there is no characteristic response that allows for a categorical conclusion about the effect of these changes on functional impairments and performance limitations. Despite the lack of uniformity in findings during fatigue, there is strong evidence for marked 'inhibition' of motoneurones as a direct result of voluntary drive. Endogenous forms of neuromodulation, such as via serotonin released from neurones, can directly affect motoneuronal output and central fatigue. Exogenous forms of neuromodulation, such as brain stimulation, may achieve a similar effect, although the evidence is weak. Non-invasive transcranial direct current stimulation can cause transient or long-lasting changes in cortical excitability; however, variable results across studies cast doubt on its claimed capacity to enhance performance. Furthermore, with these studies, it is difficult to establish a cause-and-effect relationship between brain responsiveness and exercise performance. This review briefly summarizes changes in the corticomotoneuronal pathway during various types of exercise, and considers the relevance of these changes for the development of central fatigue, as well as the potential of non-invasive brain stimulation to enhance motor cortical excitability, motoneuronal output and, ultimately, exercise performance.
Keywords: central nervous system; motor cortex; muscle fatigue; spinal motoneurones.
© 2022 The Authors. The Journal of Physiology © 2022 The Physiological Society.
Conflict of interest statement
Competing interests
The authors declare no conflict of interest.
Figures

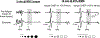
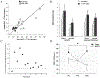
Comment in
-
Critical considerations on tDCS-mediated changes in corticospinal response to fatiguing exercise.J Physiol. 2023 Feb;601(3):703-704. doi: 10.1113/JP284152. Epub 2023 Jan 4. J Physiol. 2023. PMID: 36536518 No abstract available.
-
Reply to 'Critical considerations on tDCS-mediated changes in corticospinal response to fatiguing exercise'.J Physiol. 2023 Feb;601(3):705. doi: 10.1113/JP284271. Epub 2023 Jan 12. J Physiol. 2023. PMID: 36634118 No abstract available.
-
Critical considerations on tDCS-induced changes in corticospinal excitability and exercise performance: should we go beyond M1?J Physiol. 2023 Dec;601(23):5453-5455. doi: 10.1113/JP285507. Epub 2023 Oct 3. J Physiol. 2023. PMID: 37786946 No abstract available.
References
-
- Cengiz B, Murase N & Rothwell JC. (2013). Opposite effects of weak transcranial direct current stimulation on different phases of short interval intracortical inhibition (SICI). Exp Brain Res 225, 321–331. - PubMed
-
- Chen R, Lozano AM & Ashby P. (1999). Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128, 539–542. - PubMed
-
- Cogiamanian F, Marceglia S, Ardolino G, Barbieri S & Priori A. (2007). Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci 26, 242–249. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

