Interventions for the long-term prevention of hereditary angioedema attacks
- PMID: 36326435
- PMCID: PMC9632406
- DOI: 10.1002/14651858.CD013403.pub2
Interventions for the long-term prevention of hereditary angioedema attacks
Abstract
Background: Hereditary angioedema (HAE) is a serious and potentially life-threatening condition that causes acute attacks of swelling, pain and reduced quality of life. People with Type I HAE (approximately 80% of all HAE cases) have insufficient amounts of C1 esterase inhibitor (C1-INH) protein; people with Type II HAE (approximately 20% of all cases) may have normal C1-INH concentrations, but, due to genetic mutations, these do not function properly. A few people, predominantly females, experience HAE despite having normal C1-INH levels and C1-INH function (rare Type III HAE). Several new drugs have been developed to treat acute attacks and prevent recurrence of attacks. There is currently no systematic review and meta-analysis that included all preventive medications for HAE.
Objectives: To assess the benefits and harms of interventions for the long-term prevention of HAE attacks in people with Type I, Type II or Type III HAE.
Search methods: We used standard, extensive Cochrane search methods. The latest search date was 3 August 2021.
Selection criteria: We included randomised controlled trials in children or adults with HAE that used medications to prevent HAE attacks. The comparators could be placebo or active comparator, or both; approved and experimental drug trials were eligible for inclusion. There were no restrictions on dose, frequency or intensity of treatment. The minimum length of four weeks of treatment was required for inclusion; this criterion excluded the acute treatment of HAE attacks.
Data collection and analysis: We used standard Cochrane methods. Our primary outcomes were 1. HAE attacks (number of attacks per person, per population) and change in number of HAE attacks; 2. mortality and 3. serious adverse events (e.g. hepatic dysfunction, hepatic toxicity and deleterious changes in blood tests). Our secondary outcomes were 4. quality of life; 5. severity of breakthrough attacks; 6. disability and 7. adverse events (e.g. weight gain, mild psychological changes and body hair). We used GRADE to assess certainty of evidence for each outcome.
Main results: We identified 15 studies (912 participants) that met the inclusion criteria. The studies included people with Type I and II HAE. The studies investigated avoralstat, berotralstat, subcutaneous C1-INH, plasma-derived C1-INH, nanofiltered C1-INH, recombinant human C1-INH, danazol, and lanadelumab for the prevention of HAE attacks. We did not find any studies on the use of tranexamic acid for prevention of HAE attacks. All drugs except avoralstat reduced the number of HAE attacks compared with placebo. For breakthrough attacks that occurred despite prophylactic treatment, intravenous and subcutaneous forms of C1-INH and lanadelumab reduced attack severity. It is not known whether other drugs have a similar effect, as the severity of breakthrough attacks in people taking drugs other than C1-INH and lanadelumab was not reported. For quality of life, avoralstat, berotralstat, C1-INH (all forms) and lanadelumab increased quality of life compared with placebo; there were no data for danazol. Four studies reported on changes in disability during treatment with C1-INH, berotralstat and lanadelumab; all three drugs decreased disability compared with placebo. Adverse events, including serious adverse events, did not occur at a rate higher than placebo. However, serious adverse event data and other adverse event data were not available for danazol, which prevented us from drawing conclusions about the absolute or relative safety of this drug. No deaths were reported in the included studies. The analysis was limited by the small number of studies, the small number of participants in each study and the lack of data on older drugs, therefore the certainty of the evidence is low. Given the rarity of HAE, it is not surprising that drugs were rarely directly compared, which does not allow conclusions on the comparative efficacy of the various drugs for people with HAE. Finally, we did not identify any studies that included people with Type III HAE. Therefore, we cannot draw any conclusions about the efficacy or safety of any drug in people with this form of HAE.
Authors' conclusions: The available data suggest that berotralstat, C1-INH (subcutaneous, plasma-derived, nanofiltered and recombinant), danazol and lanadelumab are effective in lowering the risk or incidence (or both) of HAE attacks. In addition, C1-INH and lanadelumab decrease the severity of breakthrough attacks (data for other drugs were not available). Avoralstat, berotralstat, C1-INH (all forms) and lanadelumab increase quality of life and do not increase the risk of adverse events, including serious adverse events. It is possible that danazol, subcutaneous C1-INH and recombinant human C1-INH are more effective than berotralstat and lanadelumab in reducing the risk of breakthrough attacks, but the small number of studies and the small size of the studies means that the certainty of the evidence is low. This and the lack of head-to-head trials prevented us from drawing firm conclusions on the relative efficacy of the drugs.
Trial registration: ClinicalTrials.gov NCT02870972 NCT03485911 NCT03873116 NCT02586805 NCT01912456 NCT02316353 NCT01005888 NCT01756157 NCT02052141 NCT02247739 NCT01984788 NCT02303626 NCT02584959 NCT00462709 NCT03712228 NCT04656418.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
NB: none.
MF: none.
ES: none.
PM: none.
CK: has conducted original investigator‐led research in the field of hereditary angioedema (HAE) and has participated as a principal investigator in sponsored multinational randomised controlled trials (RCTs) of several therapies used for HAE management (acute and prophylactic therapies). She has participated actively in several global HAE meetings and has chaired advisory boards discussing HAE management in Australia. CK has declared that her institution has received payment for the conduct of multinational clinical trials from CSL Behring, KalVista Pharmaceuticals and BioCryst Pharmaceuticals Inc. She has received honoraria from CSL Behring and Takeda Pharmaceutical Company for serving on advisory boards and for lectures and presentations. She is a board member of HAE Australasia (patient organisation).
KM: has declared that she conducts comparative effectiveness work for TruDataRx Inc, which provides independent advice to employers based on the efficacy of pharmaceutical drugs. This company does not receive funds from the pharmaceutical industry or government.
Figures
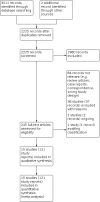










































Update of
- doi: 10.1002/14651858.CD013403
References
References to studies included in this review
APeX‐1 {published data only}
-
- Aygören‑Pürsün E, Bygum A, Grivcheva‑Panovska V, Magerl M, Graff J, Steiner UC, et al. Oral plasma kallikrein inhibitor for prophylaxis in hereditary angioedema. New England Journal of Medicine 2018;379(4):352-62. - PubMed
-
- Bygum A, Panovska VG, Maurer M, Magerl M, Farkas H, Cicardi M, et al. Pharmacokinetic (PK) and pharmacodynamic (PD) effects of BCX7353 in patients with hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE): results from APeX-1 study. Allergy 2018;73(Suppl 105):723.
-
- Farkas H, Aygoren-Pursun E, Panovska VG, Huissoon A, Kinaciyan T, Murray S, et al. Relationship of target concentrations with efficacy: results from the APeX-1 study of Bcx7353. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S33.
-
- Magerl M, Rae W, Aygoren-Pursun E, Bygum A, Panovska VG, Steiner UC, et al. BCX7353 improves health-related quality of life in hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE): findings from the APeX-1 study. Allergy 2018;73(Suppl 105):724.
-
- Steiner UC, Huissoon A, Bygum A, Panovska VG, Gompels M, Cancian M, et al. Analysis of gastrointestinal (GI) symptoms in patients with hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE) treated with BCX7353: results from APeX-1 study. Allergy 2018;73(Suppl 105):724-5.
APeX‐2 {published data only}
-
- Anderson J, Gagnon R, Best J, Murray S, Iocca H, Sitz K. Reduction in attacks in hereditary angioedema (HAE) with berotralstat is consistent regardless of prior prophylactic treatment: a subgroup analysis of the phase 3 APeX-2 trial. Journal of Allergy and Clinical Immunology 2021;147(2):AB23. [DOI: 10.1016/j.jaci.2020.12.122] - DOI
-
- Aygören‑Pürsün E, Johnston D, Banerji A, Bernstein JA, Yang WH, Fritz S, et al. Berotralstat (BCX7353) treatment demonstrates robust and durable reduction in the rate of hereditary angioedema (HAE) attacks over 48 weeks of the phase 3 APeX-2 study. European Journal of Allergy and Clinical Immunology 2020;75(Suppl 109):457-8. [DOI: 10.1111/all.14508] - DOI
-
- Gower R, Busse P, Best J, Murray S, Iocca H, Kinaciyan T. Berotralstat reduces use of on-demand medication in hereditary angioedema (HAE) patients previously treated with prophylactic therapies. Journal of Allergy and Clinical Immunology 2021;147(2 Suppl):AB146.
-
- Jacobs J, Aygören-Pürsün E, Sitz K, Tachdjian R, Li H, Best J, et al. Berotralstat positively impact patient-reported satisfaction: results from the phase 3 APeX-2 trial. Annals of Allergy, Asthma & Immunology 2020;125(5 Suppl):S25.
-
- Johnston D, Banerji A, Riedl M, Soteres D, Berstein J, Best J, et al. Berotralstat improves patient-reported quality of life through 48 weeks in the phase 3 APeX-2 trial. Annals of Allergy, Asthma & Immunology 2020;125(5 Suppl):S22.
APeX‐J {published data only}
Banerji 2017 {published data only}
-
- Banerji A, Busse P, Shennak M, Lumry W, Davis‑Lorton M, Wedner HJ, et al. Inhibiting plasma kallikrein for hereditary angioedema prophylaxis. New England Journal of Medicine 2017;376(8):717-28. - PubMed
-
- Banerji A, Busse P, Shennak M, Lumry W, Davis-Lorton M, Wedner J, et al. Interim analysis results of a phase 1b, multiple ascending dose study to evaluate DX-2930, a fully human monoclonal antibody inhibitor of plasma kallikrein in development for long-term prophylaxis of hereditary angioedema. Allergy 2015;70(S101):107.
-
- Busse P, Banerji A, Shennak M, Lumry W, Davis-Lorton M, Wedner H, et al. Dx-2930 in patients with hereditary angioedema: final results of a phase 1b study. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl):A31.
-
- Jacobs JS, Busse PJ, Banerji A, Shennak M, Lumry WR, Davis-Lorton MA, et al. Relationship between drug exposure and clinical response observed in the phase 1b study of DX-2930 in subjects with hereditary angioedema. Journal of Allergy and Clinical Immunology 2016;137(2 Suppl):AB251.
-
- Lumry W, Cicardi M, Busse P, Banerji A, Shennak M, Davis-Lorton M, et al. Attack frequency, C1-INH function, and levels of cleaved kininogen do not influence the clinical response to DX-2930 in patients with hereditary angioedema. Annals of Allergy, Asthma & Immunology 2015;115(5 Suppl):A31.
COMPACT {published data only}
-
- Anderson J, Krishnarajah G, Craig T, Lumry W, Supina D, Feuersenger H, et al. Use of rescue medication in hereditary angioedema attacks and its relation to attack severity. Annals of Allergy, Asthma & Immunology 2017;119(5 Suppl 1):S40.
-
- Christiansen S, Zuraw B, Feuersenger H, Pragst I, Pawaskar D. C1-Esterase inhibitor (C1-INH) functional activity of subcutaneous C1-INH for the prevention of hereditary angioedema (HAE) attacks: pharmacokinetic comparison in adolescent and adult patients. Allergy 2018;73(Suppl 105):613-4.
-
- Cicardi M, Zuraw B, Craig T, Longhurst H, Feuersenger H, Jacobs I, et al. Onset of effect of prophylactic treatment with subcutaneous C1-esterase inhibitor (C1-INH [SC]) for prevention of hereditary angioedema (HAE) attacks: findings from the phase III compact study. Allergy 2018;73(Suppl 105):615-6.
-
- Craig T, Lumry W, Cicardi M, Zuraw B, Bernstein JA, Anderson J, et al. Treatment effect of switching from intravenous to subcutaneous C1-inhibitor for prevention of hereditary angioedema attacks: COMPACT subgroup findings. Journal of Allergy and Clinical Immunology. In Practice 2019;7(6):2035-8. - PubMed
-
- Craig T, Zuraw B, Cicardi M, Longhurst H, Chiao J, Feuersenger H, et al. Prevention of hereditary angioedema (HAE) attacks by anatomical location with subcutaneous C1-esterase inhibitor (C1-INH [SC]) treatment: results from the phase III COMPACT study. Allergy 2018;73(Suppl 105):615.
COMPACT extension {published data only}
-
- Bernstein JA, Schwartz L, Yang W, Baker J, Anderson J, Farkas H, et al. Long-term safety and efficacy of subcutaneous C1-inhibitor in older patients with hereditary angioedema. Annals of Allergy, Asthma & Immunology 2020;125(3):334-40. - PubMed
-
- Christiansen S, Li H, Chiao J, Jacobs I. Comparison of 40 IU/kg and 60 IU/kg doses of subcutaneous C1-esterase inhibitor (C1-INH [SC]) for the prophylactic treatment of hereditary angioedema (HAE): efficacy results from a phase 3 trial. Allergy and Asthma Proceedings 2017;38(5):397.
-
- Craig T, Longhurst H, Cicardi M, Zuraw B. Safety and efficacy of long-term subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. Annals of Allergy, Asthma & Immunology 2018;121(Suppl 5):S34. - PubMed
-
- Craig T, Zuraw B, Longhurst H, Cicardi M, Bork K, Grattan C, et al. Long-term outcomes with subcutaneous C1-inhibitor replacement therapy for prevention of hereditary angioedema attacks. Journal of Allergy and Clinical Immunology. In Practice 2019;7(6):1793-802.e2. - PubMed
-
- Katelaris C, Prusty S. Efficacy and safety of subcutaneous C1-inhibitor in Australian patients with hereditary angioedema. Internal Medicine Journal 2019;49(Suppl S4):27.
Gelfand 1976 {published data only}
-
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol: reversal of clinical and biochemical abnormalities. New England Journal of Medicine 1976;295(26):1444-8. - PubMed
HELP {published data only}
-
- Banerji A, Riedl M, Bernstein J, Cicardi M, Longhurst H, Zuraw B, et al. Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 HELP study. Annals of Allergy, Asthma & Immunology 2017;119(5 Suppl):S5.
-
- Banerji A, Riedl M, Zuraw B, Lumry W, Lu P, Hao J, et al. Lanadelumab 300mg every 2 weeks effectively prevented hereditary angioedema attacks in the HELP study. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S5.
-
- Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Lanadelumab for prevention of attacks in hereditary angioedema: results from the phase 3 HELP study. Swiss Medical Weekly 2018;148(Suppl 231):28S-9S.
-
- Bernstein JA, Banerji A, Schranz J, Nurse C, Riedl M, Lu P. Lanadelumab reduces high morbidity attacks in patients with hereditary angioedema (HAE): results from the phase 3 HELP study. Allergy 2018;73(Suppl 105):288-89.
NCT01005888 {published data only}
-
- Bernstein J, Manning M, Li H, White MV, Baker J, Lumry WR, et al. Safety and efficacy of escalating doses of C1 esterase inhibitor [human] as prophylaxis in patients with hereditary angioedema (HAE). Annals of Allergy, Asthma & Immunology 2012;109(5):A29. - PubMed
-
- Bernstein JA, Li HH, Craig TJ, Longhurst HJ, Farkas H, Manning ME, et al. Placebo-controlled trials of C1-inhibitor (C1-INH) replacement therapy for routine prevention of attacks in patients with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB234.
-
- Dayno J, Miller DP, Hautamaki E, Newcomer S, Fitts D, Lumry WR. Relationship between angioedema attacks and health-related quality of life outcomes in patients with hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2014;133(2 Suppl):AB33.
-
- Lumry W, Manning ME, Hurewitz DS, Davis-Lorton M, Fitts D, Kalfus IN, et al. Nanofiltered C1-esterase inhibitor for the acute management and prevention of hereditary angioedema attacks due to C1-inhibitor deficiency in children. Journal of Paediatrics 2013;162(5):1017-22.e1. - PubMed
-
- Lumry WR, Miller DP, Beusterien K, Hautamaki E, Fitts D, Dayno J. Health-related quality of life (HRQoL) in patients with hereditary angioedema (HAE) receiving nanofiltered C1 inhibitor (C1 INH-nf) for prophylaxis: results of a randomised, placebo-controlled, cross-over study. Allergy 2014;69(S99):491.
NCT01756157 {published data only}
-
- Lumry WR, Li HH, Magerl M, Maurer M, Bernstein JA, Riedl MA, et al. Pharmacokinetics of subcutaneous C1 esterase inhibitor (human) with recombinant human hyaluronidase for the prevention of angioedema attacks in patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB192.
-
- Reshef A, Riedl M, Panovska VG, Moldovan D, Baker J, Yang WH, et al. Randomized, double-blind, placebo-controlled trial of recombinant human C1 inhibitor for prophylaxis of hereditary angioedema attacks. World Allergy Organization Journal 2017;10(Suppl 1):A106.
-
- Riedl M, Grivcheva-Panovska V, Moldovan D, Baker J, Yang WH, Reshef A, et al. Randomized, double-blind, placebo-controlled trial of recombinant human C1 inhibitor for prophylaxis of hereditary angioedema attacks. Allergy and Asthma Proceedings 2017;38(3):237.
-
- Riedl MA, Lumry WR, Li HH, Banerji A, Bernstein JA, Ba M, et al. Subcutaneous administration of human C1 inhibitor with recombinant human hyaluronidase in patients with hereditary angioedema. Allergy and Asthma Proceedings 2016;37(6):489-500. - PubMed
-
- Weller K, Maurer M, Fridman M, Supina D, Schranz J, Magerl M. Health-related quality of life with hereditary angioedema following prophylaxis with subcutaneous C1-inhibitor with recombinant hyaluronidase. Allergy and Asthma Proceedings 2017;38(2):143-51. - PubMed
NCT02052141 {published data only}
-
- Aygören-Pürsün E, Jacobson K, Moldovan D, Christensen J, Leerberghe A, Wang Y, et al. Safety and efficacy of a C1 inhibitor for the prevention of hereditary angioedema attacks in children: interim results of a phase 3 study. Allergy, Asthma and Clinical Immunology 2017;13(Suppl 2):O-14.
-
- Farkas H, Aygoren-Pursun E, Martinez-Saguer I, Kessel A, Hao J, Lu P, et al. The use of a C1 esterase inhibitor concentrate to manage hereditary angioedema attacks in children. Allergy 2017;72(Suppl 103):101.
-
- Jacobson K, Soteres D, Nieto-Martinez S, Moldovan D, Martinez-Saguer I, Vardi M, et al. C1 inhibitor administration in pediatric patients with hereditary angioedema: patient compliance with intravenous therapy. Annals of Allergy, Asthma & Immunology 2018;121(5 Suppl):S36.
-
- Martinez-Saguer I, Soteres D, Leerberghe A, Herrera EM, Devercelli G, Vardi M, et al. Improved health-related quality of life in pediatric patients with hereditary angioedema (HAE): a phase 3 study of C1 inhibitor for attack prevention. Allergy 2018;73(Suppl 105):718.
NCT02247739 {published data only}
-
- Riedl MA, Grivcheva-Panovska V, Moldovan D, Baker J, Yang WH, Giannetti BM, et al. Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio-oedema: a phase 2, multicentre, randomised, double-blind, placebo-controlled crossover trial. Lancet 2017;390(10102):1595-602. - PubMed
-
- Yang WH, Li HH, Chase C, Moldovan D, Grivcheva-Panovska V, Harper JR, et al. Recombinant Human C1 Inhibitor (rHC1INH) Is efficacious and well tolerated as prophylaxis for prevention of hereditary angioedema (HAE) attacks: a randomized, phase 2 trial. Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB231.
OPuS‐1 {published data only}
-
- Aygören-Pürsün E, Magerl M, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. Efficacy correlates with plasma levels in OPuS-1, a proof-of-concept study of oral kallikrein inhibitor BCX4161 as a prophylaxis against attacks of hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2015;135(Suppl 1):AB192.
-
- Aygören-Pürsün E, Magerl M, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. Prophylaxis of hereditary angioedema attacks: a randomized trial of oral plasma kallikrein inhibition with avoralstat. Journal of Allergy and Clinical Immunology 2016;138(3):934-6.e5. - PubMed
-
- Magerl M, Aygören-Pürsün E, Graff J, Martinez-Saguer I, Kreuz W, Longhurst H, et al. BCX4161, an oral kallikrein inhibitor, showed significant benefits on reducing disease burden and improving quality of life in subjects with hereditary angioedema in the OPuS-1 study. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB278.
-
- Magerl M, Rae W, Aygoren-Pursun E, Bygum A, Panovska VG, Steiner UC, et al. Prophylactic therapy with BCX7353 improves anxiety and stress in hereditary angioedema with C1-inhibitor deficiency (C1-INH-HAE) patients. Allergy 2018;73(Suppl 105):414.
OPuS‐2 {published data only}
SAHARA {published data only}
-
- Farkas H, Martinez-Saguer I, Yang WH, Johnston D, Tang T, Vardi M, et al. Reduction of attack severity with fixed-dose subcutaneous (SC) C1 inhibitor liquid in hereditary angioedema patients: results from the phase 3 SAHARA study. Allergy 2018;73(Suppl 105):720-1.
-
- Lumry W, Bernstein JA, Jacobs J, Yang WH, Moldovan D, Riedl MA, et al. Fixed-dose subcutaneous (SC) C1 inhibitor liquid for prophylactic treatment of hereditary angioedema attacks: results from the phase 3 SAHARA study. Allergy 2018;73(Suppl 105):287.
-
- Lumry W, Bernstein JA, Jacobs J, Yang WH, Moldovan D, Riedl MA, et al. Fixed-dose subcutaneous (SC) C1 inhibitor liquid for prophylactic treatment of hereditary angioedema attacks: results from the phase 3 SAHARA study. Swiss Medical Weekly 2018;148(Suppl 231):26S-7S.
-
- Lumry WR, Martinez-Saguer I, Yang WH, Bernstein JA, Jacobs J, Moldovan D, et al. Fixed-dose subcutaneous C1-inhibitor liquid for prophylactic treatment of C1-INH-HAE: SAHARA randomized study. Journal of Allergy and Clinical Immunology. In Practice 2019;7(5):1610-18.e4. - PubMed
References to studies excluded from this review
Aabom 2015 {published data only}
-
- Aabom A, Andersen KE, Perez-Fernandez E, Caballero T, Bygum A. Health-related quality of life in Danish patients with hereditary angioedema. Acta Dermato-venereologica 2015;95(2):225-6. - PubMed
Aberer 2017 {published data only}
-
- Aberer W, Maurer M, Bouillet L, Zanichelli A, Caballero T, Longhurst HJ, et al. Breakthrough attacks in patients with hereditary angioedema receiving long-term prophylaxis are responsive to icatibant: findings from the Icatibant Outcome Survey. Allergy, Asthma, and Clinical Immunology 2017;13:31. [DOI: 10.1186/s13223-017-0203-z] - DOI - PMC - PubMed
Agostoni 1978a {published data only}
-
- Agostoni A, Marasini B, Cicardi M, Martignoni GC. Intermittent therapy with danazol in hereditary angioedema. Lancet 1978;1(8061):453. - PubMed
Agostoni 1978b {published data only}
-
- Agostoni A, Marasini B, Cicardi M, Martignoni G, Uziel L, Pietrogrande M. Hepatic function and fibrinolysis in patients with hereditary angioedema undergoing long-term treatment with tranexamic acid. Allergy 1978;33(4):216-21. - PubMed
Agostoni 1980a {published data only}
-
- Agostoni A, Cicardi M, Martignoni GC, Bergamaschini L, Marasini B. Danazol and stanozolol in long-term prophylactic treatment of hereditary angioedema. Journal of Allergy and Clinical Immunology 1980;65(1):75-9. - PubMed
Agostoni 1983 {published data only}
-
- Agostoni A. The management of hereditary angioedema. Ricerca in Clinica e in Laboratorio 1983;13(1):55-9. - PubMed
Aygören‐Pürsün 2013 {published data only}
Baker 2013 {published data only}
-
- Baker JW, Craig TJ, Riedl MA, Banerji A, Fitts D, Kalfus IN, et al. Nanofiltered C1 esterase inhibitor (human) for hereditary angioedema attacks in pregnant women. Allergy and Asthma Proceedings 2013;34(2):162-9. - PubMed
Bernstein 2019 {published data only}
-
- Bernstein JA, Li HH, Craig TJ, Manning ME, Lawo JP, Machnig T, et al. Indirect comparison of intravenous vs. subcutaneous C1-inhibitor placebo-controlled trials for routine prevention of hereditary angioedema attacks. Allergy, Asthma, and Clinical Immunology 2019;15:13. [DOI: 10.1186/s13223-019-0328-3] - DOI - PMC - PubMed
Birjmohun 2008 {published data only}
-
- Birjmohun RS, Hovingh GK, Stroes ES, Hofstra JJ, Dallinga-Thie GM, Meijers JC, et al. Effects of short-term and long-term danazol treatment on lipoproteins, coagulation, and progression of atherosclerosis: two clinical trials in healthy volunteers and patients with hereditary angioedema. Clinical Therapeutics 2008;30(12):2314-23. - PubMed
Blohmé 1972 {published data only}
-
- Blohmé G. Treatment of hereditary angioneurotic oedema with tranexamic acid. A random double-blind cross-over study. Acta Medica Scandinavica 1972;192:293-8. - PubMed
Bork 2008 {published data only}
-
- Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Annals of Allergy, Asthma & Immunology 2008;100(2):153-61. - PubMed
Bork 2011 {published data only}
-
- Bork K, Hardt J. Hereditary angioedema: long-term treatment with one or more injections of C1 inhibitor concentrate per week. International Archives of Allergy and Immunology 2011;154(1):81-8. - PubMed
Bork 2017 {published data only}
-
- Bork K, Wulff K, Witzke G, Hardt J. Treatment for hereditary angioedema with normal C1-INH and specific mutations in the F12 gene (HAE-FXII). Allergy 2017;72(2):320-4. - PubMed
Busse 2017 {published data only}
-
- Busse P, Baker J, Martinez-Saguer I, Bernstein JA, Craig T, Magerl M, et al. Safety of C1-inhibitor concentrate use for hereditary angioedema in pediatric patients. Journal of Allergy and Clinical Immunology. In Practice 2017;5(4):1142-5. - PubMed
Chyung 2014 {published data only}
-
- Chyung Y, Vince B, Iarrobino R, Sexton D, TenHoor C, Kenniston J, et al. Results of a phase 1a, single ascending dose study of DX-2930, a human monoclonal antibody inhibitor of plasma kallikrein under investigation for long term prophylaxis of hereditary angioedema. Allergy 2014;69(S99):581.
Cicardi 1997 {published data only}
-
- Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. Journal of Allergy and Clinical Immunology 1997;2:194-6. - PubMed
Davis‐Lorton 2016 {published data only}
-
- Davis-Lorton MA, Busse PJ, Banerji A, Shennak M, Lumry WR, Wedner HJ, et al. Pharmacodynamic effect of DX-2930 on plasma kallikrein in hereditary angioedema patients. Journal of Allergy and Clinical Immunology 2016;137(2):AB252.
Drouet 2008 {published data only}
EudraCT 2009‐010736‐18 {published data only}
-
- EudraCT 2009-010736-18. An open-label exploratory phase II study of the safety and prophylactic effect of a weekly 50 U/kg rC1INH treatment in asymptomatic patients with hereditary C1INH deficiency (HAE) – OPERA. clinicaltrialsregister.eu/ctr-search/trial/2009-010736-18/HU (first received 4 April 2009).
EudraCT 2010‐019670‐32 {published data only}
-
- EudraCT 2010-019670-32. Pharmacokinetics and safety of human pasteurised C1-inhibitor concentrate (Berinert/CE1145) in subjects with congenital C1-INH deficiency. clinicaltrialsregister.eu/ctr-search/trial/2010-019670-32/IT (first received 19 December 2012).
Farkas 2010 {published data only}
-
- Farkas H, Czaller I, Csuka D, Vas A, Valentin S, Varga L, et al. The effect of long-term danazol prophylaxis on liver function in hereditary angioedema – a longitudinal study. European Journal of Clinical Pharmacology 2010;66(4):419-26. - PubMed
Farkas 2013 {published data only}
-
- Farkas H, Csuka D, Zotter Z, Varga L, Fust G. Prophylactic therapy in children with hereditary angioedema. Journal of Allergy and Clinical Immunology 2013;131(2):579-82.e1-2. - PubMed
Füst 2011 {published data only}
-
- Füst G, Farkas H, Csuka D, Varga L, Bork K. Long-term efficacy of danazol treatment in hereditary angioedema. European Journal of Clinical Investigation 2011;41(3):256-62. - PubMed
Hofstra 2012 {published data only}
-
- Hofstra JJ, Kleine Budde I, Twuyver E, Choi G, Levi M, Leebeek FW, et al. Treatment of hereditary angioedema with nanofiltered C1-esterase inhibitor concentrate (Cetor®): multi-center phase II and III studies to assess pharmacokinetics, clinical efficacy and safety. Clinical Immunology (Orlando, Fla.) 2012;142(3):280-90. - PubMed
NCT01108848 {published data only}
-
- NCT01108848. Patient registry study of Berinert® in normal clinical practice. clinicaltrials.gov/show/NCT01108848 (first received 22 April 2010).
NCT01467947 {published data only}
-
- NCT01467947. Postmarketing immunogenicity study in HAE subjects treated with Berinert. clinicaltrials.gov/show/NCT01467947 (first received 9 November 2011).
NCT01576523 {published data only}
-
- NCT01576523. A study to evaluate the clinical pharmacology and safety of C1-esterase inhibitor administered by the subcutaneous route. clinicaltrials.gov/show/NCT01576523 (first received 12 April 2012).
NCT01760343 {published data only}
-
- NCT01760343. A study to evaluate the safety and pharmacokinetics of two formulations of C1-esterase inhibitor. clinicaltrials.gov/show/NCT01760343 (first received 4 January 2013).
Sharma 2009 {published data only}
-
- Sharma K, Levy RJ, Wasserman RL, Jacobson KW, Laudadio C, Craig TJ. Evaluation of prodromal symptoms concurrent with hereditary angioedema therapy. Journal of Allergy and Clinical Immunology 2009;123(2 Suppl):S100.
Sweet 1980 {published data only}
-
- Sweet LC, Jackson CE, Yanari SS, Yott JB. Danazol therapy in hereditary angioedema. Henry Ford Hospital Medical Journal 1980;28(1):31-5. - PubMed
Szegedi 2008 {published data only}
-
- Szegedi R, Széplaki G, Varga L, Prohaszka Z, Szeplaki Z, Karadi I, et al. Long-term danazol prophylaxis does not lead to increased carotid intima-media thickness in hereditary angioedema patients. Atherosclerosis 2008;198(1):184-91. - PubMed
Széplaki 2005 {published data only}
-
- Széplaki G, Varga L, Valentin S, Kleiber M, Karadi I, Romics L, et al. Adverse effects of danazol prophylaxis on the lipid profiles of patients with hereditary angioedema. Journal of Allergy and Clinical Immunology 2005;115(4):864-9. - PubMed
Wang 2017 {published data only}
-
- Wang Y, Martin P, Lu P, Schranz J. Pharmacokinetics (PK) and pharmacodynamics (PD) of c1 esterase inhibitor (c1-IHN) for prophylaxis of angioedema attacks in patients (PTS) with hereditary angioedema (HAE). Allergy 2017;72(Suppl 103):148.
Waytes 1996 {published data only}
-
- Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. New England Journal of Medicine 1996;334(25):1630-4. - PubMed
Zotter 2013 {published data only}
-
- Zotter Z, Czaller I, Csuka D, Szabo E, Kohalmi KV, Varga L, et al. Adverse effects of danazol prophylaxis in female patients with hereditary angioedema due to C1-INH deficiency (HAE-C1-INH). Allergy: European Journal of Allergy and Clinical Immunology 2013;97:430.
References to studies awaiting assessment
Zhang 1990 {published data only}
-
- Zhang H. Long-term therapy of hereditary angioedema. Chung-Hua i Hsueh Tsa Chih 1990;70(4):211-3. - PubMed
References to ongoing studies
NCT03712228 {published data only}
-
- NCT03712228. A study to investigate CSL312 in subjects with hereditary angioedema (HAE). clinicaltrials.gov/ct2/show/NCT03712228 (first received 19 October 2018).
NCT04656418 {published data only}
-
- NCT04656418. CSL312 (Garadacimab) in the prevention of hereditary angioedema attacks. clinicaltrials.gov/ct2/show/NCT04656418 (first received 7 December 2020).
Additional references
Agostoni 1999
-
- Agostoni A, Cicardi M, Cugno M, Zingale LC, Gioffre D, Nussberger J. Angioedema due to angiotensin-converting enzyme inhibitors. Immunopharmacology 1999;44:21-5. - PubMed
ASCIA 2020
-
- Australasian Society of Clinical Immunology and Allergy. Hereditary angioedema (HAE) position paper. allergy.org.au/images/docs/ASCIA_HP_Position_Paper_HAE_2020_Aug_Update.pdf (accessed 13 May 2021).
Bafunno 2018
-
- Bafunno V, Firinu D, D'Apolito M, Cordisco G, Loffredo S, Leccese A, et al. Mutation of the angiopoietin-1 gene (ANGPT1) associates with a new type of hereditary angioedema. Journal of Allergy and Clinical Immunology 2018;141(3):1009-17. - PubMed
Banerji 2018
Bork 2018
-
- Bork K, Wulff K, Steinmüller-Magin L, Braenne I, Staubach-Renz P, Witzke G, et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy 2018;73(2):442-50. - PubMed
Brigden 2018
Cao 2015
-
- Cao Z, Biondo M, Rayzman V, Hardy M, McDonald A, Busfield S, et al. Development and characterization of an anti-FXIIa monoclonal antibody for the treatment of hereditary angioedema. Journal of Allergy and Clinical Immunology 2015;135(2 Suppl):AB194.
Chen 2017
-
- Chen X, Kotian P, Wilson R, Parker CD, Babu YS. Preclinical characterization of BCX7353, an oral plasma kallikrein inhibitor, for the treatment of hereditary angioedema (HAE). Journal of Allergy and Clinical Immunology 2017;139(2 Suppl):AB230.
Cicardi 1997
-
- Cicardi M, Castelli R, Zingale LC, Agostoni A. Side effects of long-term prophylaxis with attenuated androgens in hereditary angioedema: comparison of treated and untreated patients. Journal of Allergy and Clinical Immunology 1997;99:194-6. - PubMed
Cohen 1988
-
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd edition. New York (NY): Taylor & Francis, 1988. [DOI: 10.4324/9780203771587] [ISBN: 9780203771587] - DOI
Fabiani 1990
-
- Fabiani JE, Paulin P, Simkin G, Leoni J, Palombarani S, Squiquera L. Hereditary angioedema: therapeutic effect of danazol on C4 and C1 esterase inhibitors. Annals of Allergy 1990;64(4):388-92. - PubMed
FDA 2011
-
- Food and Drug Administration. Danocrine label ID: 3061327. www.accessdata.fda.gov/drugsatfda_docs/label/2011/017557s033s039s040s041... (accessed 20 December 2018):1-9.
Frank 1976
-
- Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Annals of Internal Medicine 1976;84:580-93. - PubMed
Frank 1979
-
- Frank MM. Effect of sex hormones on the complement-related clinical disorder of hereditary angioedema. Arthritis and Rheumatism 1979;22:1295-99. - PubMed
Frank 2016
-
- Frank MM, Zuraw B, Banerji A, Bernstein JA, Craig T, Busse P, et al. Management of children with hereditary angioedema due to C1 inhibitor deficiency. Pediatrics 2016;138(5):e20160575. - PubMed
Frese 2019
Germenis 2016
-
- Germenis AE, Speletas M. Genetics of hereditary angioedema revisited. Clinical Reviews in Allergy and Immunology 2016;51(2):170-82. - PubMed
Gompels 2002
GRADE 2004
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 20 December 2018. Hamilton, ON: McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Henao 2016
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017), Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2021
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Johnson 2018
-
- Johnson NM, Phillips MA. New treatments for hereditary angioedema. Skin Therapy Letters 2018;23(1):6-8. - PubMed
Kenniston 2014
Kunschak 1998
-
- Kunschak M, Engl W, Maritsch F, Rosen FS, Eder G, Zerlauth G, et al. A randomized, controlled trial to study the efficacy and safety of C1 inhibitor concentrate in treating hereditary angioedema. Transfusion 1998;38(6):540-9. - PubMed
Lefebvre 2021
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2.
Liberati 2009
Longhurst 2016
-
- Longhurst H, Bygum A. The humanistic, societal and pharmaco-economic burden of angioedema. Clinical Reviews in Allergy and Immunology 2016;51(2):230-9. - PubMed
Longhurst 2018
Lopes Veronez 2021
Lumry 2018
Maurer 2018
-
- Maurer M, Magerl M, Ignacio A, Emel AP, Betschel S, Bork K, et al. The international WAO/EAACI guideline for the management of hereditary angioedema – the 2017 revision and update. World Allergy Organization Journal 2018;11:5.
Norman 2003
-
- Norman GR, Sloan JA, Wyrich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Medical Care 2003;41(5):582-92. - PubMed
Review Manager 2014 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roche 2005
-
- Roche O, Blanch A, Caballero T, Sastre N, Callejo D, Lopez-Trascasa M. Hereditary angioedema due to C1 inhibitor deficiency: patient registry and approach to the prevalence in Spain. Annals of Allergy, Asthma & Immunology 2005;94(4):498-503. - PubMed
Tarzi 2007
US HAE Association 2018
-
- US HAE Association. Diagnosing HAE. www.haea.org/diagnosis.php (accessed 11 November 2018).
Weller 2016
-
- Weller K, Magerl M, Peveling-Oberhag A, Martus P, Staubach P, Maurer M. The Angioedema Quality of Life Questionnaire (AE-QoL) – assessment of sensitivity to change and minimal clinically important difference. Allergy 2016;71(8):1203-9. - PubMed
Wilson 2010
-
- Wilson DA, Bork K, Shea EP, Rentz AM, Blaustein MB, Pullman WE. Economic costs associated with acute attacks and long-term management of hereditary angioedema. Annals of Allergy, Asthma and Immunology 2010;104(4):314-20. - PubMed
Wintenberger 2014
Wyrwich 2005
Zeerleder 2016
-
- Zeerleder S, Levi M. Hereditary and acquired C1-inhibitor-dependent angioedema: from pathophysiology to treatment. Annals of Medicine 2016;48(4):256-67. - PubMed
Zotter 2014
Zuraw 2008
-
- Zuraw BL. Hereditary angioedema. New England Journal of Medicine 2008;359:1027-36. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

