Lasmiditan promotes recovery from acute kidney injury through induction of mitochondrial biogenesis
- PMID: 36326468
- PMCID: PMC9762961
- DOI: 10.1152/ajprenal.00249.2022
Lasmiditan promotes recovery from acute kidney injury through induction of mitochondrial biogenesis
Abstract
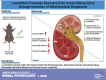
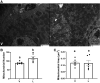
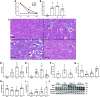
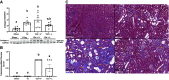
Acute kidney injury (AKI) involves rapid loss of renal function and occurs in 8-16% of hospitalized patients. AKI can be induced by drugs, sepsis, and ischemia-reperfusion (I/R). Hallmarks of AKI include mitochondrial and microvasculature dysfunction as well as renal tubular injury. There is currently no available therapeutic for AKI. Previously, our group identified that serotonin (5-HT)1F receptor agonism with lasmiditan accelerated endothelial cell recovery and induced mitochondrial biogenesis (MB) in vitro. We hypothesized that lasmiditan, a Federal Drug Administration-approved drug, would induce MB and improve microvascular and renal function in a mouse model of AKI. Male mice were subjected to renal I/R and treated with lasmiditan (0.3 mg/kg) or vehicle beginning 24 h after injury and then daily until euthanasia at 6 or 12 days. Serum creatinine was measured to estimate glomerular filtration rate. The renal cortex was assessed for mitochondrial density, vascular permeability and integrity, tubular damage, and interstitial fibrosis. Lasmiditan increased mitochondrial number (1.4-fold) in renal cortices. At 6 days, serum creatinine decreased 41% in the I/R group and 72% with lasmiditan. At 6 or 12 days, kidney injury molecule-1 increased in the I/R group and decreased 50% with lasmiditan. At 12 days, interstitial fibrosis decreased with lasmiditan by 50% and collagen type 1 by 38%. Evan's blue dye leakage increased 2.5-fold in the I/R group and was restored with lasmiditan. The tight junction proteins zonula occludens-1, claudin-2, and claudin-5 decreased in the I/R group and recovered with lasmiditan. At 6 or 12 days, peroxisome proliferator-activated receptor-γ coactivator-1α and electron transport chain complexes increased only with lasmiditan. In conclusion, lasmiditan treatment beginning AKI induces MB, attenuated vascular and tubular injury, decreased interstitial fibrosis, and lowered serum creatinine. Given that lasmiditan is a Federal Drug Administration-approved drug, these preclinical data support repurposing lasmiditan as a therapeutic for AKI.NEW & NOTEWORTHY AKI pathology involves a rapid decline in kidney function and occurs in 8-16% of hospitalized patients. There is currently no therapeutic for AKI. AKI results in mitochondria dysfunction, microvasculature injury, and loss of renal tubular function. In an I/R-induced AKI mouse model, treatment with the FDA-approved 5-HT1F receptor-selective agonist lasmiditan induced mitochondrial biogenesis, improved vascular integrity, reduced fibrosis, and reduced proximal tubule damage. These data support repurposing lasmiditan for the treatment of AKI.
Keywords: acute kidney injury; fibrosis; microvasculature injury; mitochondrial biogenesis; mitochondrial dysfunction.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
Lasmiditan induces mitochondrial biogenesis in primary mouse renal peritubular endothelial cells and augments wound healing and tubular network formation.Am J Physiol Cell Physiol. 2025 Apr 1;328(4):C1318-C1332. doi: 10.1152/ajpcell.00116.2025. Epub 2025 Mar 13. Am J Physiol Cell Physiol. 2025. PMID: 40080391 Free PMC article.
-
Lasmiditan restores mitochondrial quality control mechanisms and accelerates renal recovery after ischemia-reperfusion injury.Biochem Pharmacol. 2023 Dec;218:115855. doi: 10.1016/j.bcp.2023.115855. Epub 2023 Oct 21. Biochem Pharmacol. 2023. PMID: 37866804 Free PMC article.
-
FDA-approved 5-HT1F receptor agonist lasmiditan induces mitochondrial biogenesis and enhances locomotor and blood-spinal cord barrier recovery after spinal cord injury.Exp Neurol. 2021 Jul;341:113720. doi: 10.1016/j.expneurol.2021.113720. Epub 2021 Apr 10. Exp Neurol. 2021. PMID: 33848513 Free PMC article.
-
The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases.Biomolecules. 2020 Feb 24;10(2):347. doi: 10.3390/biom10020347. Biomolecules. 2020. PMID: 32102312 Free PMC article. Review.
-
Serotonin regulation of mitochondria in kidney diseases.Pharmacol Res. 2024 May;203:107154. doi: 10.1016/j.phrs.2024.107154. Epub 2024 Mar 22. Pharmacol Res. 2024. PMID: 38521286 Free PMC article. Review.
Cited by
-
Discovery of a Novel, Potent, and Selective 5‑Hydroxytryptamine 2B Receptor Antagonist that Induces Mitochondrial Biogenesis in the Kidney.ACS Pharmacol Transl Sci. 2025 May 30;8(6):1741-1755. doi: 10.1021/acsptsci.5c00161. eCollection 2025 Jun 13. ACS Pharmacol Transl Sci. 2025. PMID: 40534670
-
5-HT1F receptor agonism induces mitochondrial biogenesis and increases cellular function in brain microvascular endothelial cells.Front Cell Neurosci. 2024 Mar 5;18:1365158. doi: 10.3389/fncel.2024.1365158. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38510106 Free PMC article.
-
Comprehensive overview of the role of mitochondrial dysfunction in the pathogenesis of acute kidney ischemia-reperfusion injury: a narrative review.J Yeungnam Med Sci. 2024 Apr;41(2):61-73. doi: 10.12701/jyms.2023.01347. Epub 2024 Feb 14. J Yeungnam Med Sci. 2024. PMID: 38351610 Free PMC article.
-
Mitophagy regulates mitochondrial number following pharmacological induction of mitochondrial biogenesis in renal proximal tubule cells.Front Pharmacol. 2024 Feb 5;15:1344075. doi: 10.3389/fphar.2024.1344075. eCollection 2024. Front Pharmacol. 2024. PMID: 38375036 Free PMC article.
-
Research Hotspots in Mitochondria-Related Studies for AKI Treatment: A Bibliometric Study.Drug Des Devel Ther. 2024 Sep 11;18:4051-4063. doi: 10.2147/DDDT.S473426. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39280255 Free PMC article.
References
-
- Cameron JS. Allergic interstitial nephritis: clinical features and pathogenesis. Q J Med 66: 97–115, 1988. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

