KCa1.1 channels contribute to optogenetically driven post-stimulation silencing in cerebellar molecular layer interneurons
- PMID: 36326690
- PMCID: PMC9640226
- DOI: 10.1085/jgp.202113004
KCa1.1 channels contribute to optogenetically driven post-stimulation silencing in cerebellar molecular layer interneurons
Abstract
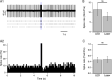
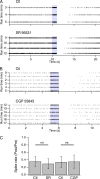
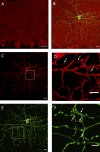
Using cell-attached recordings from molecular layer interneurons (MLI) of the cerebellar cortex of adult mice expressing channel rhodopsin 2, we show that wide-field optical activation induces an increase in firing rate during illumination and a firing pause when the illumination ends (post-stimulation silencing; PSS). Significant spike rate changes with respect to basal firing rate were observed for optical activations lasting 200 ms and 1 s as well as for 1 s long trains of 10 ms pulses at 50 Hz. For all conditions, the net effect of optical activation on the integrated spike rate is significantly reduced because of PSS. Three lines of evidence indicate that this PSS is due to intrinsic factors. Firstly, PSS is induced when the optical stimulation is restricted to a single MLI using a 405-nm laser delivering a diffraction-limited spot at the focal plane. Secondly, PSS is not affected by block of GABA-A or GABA-B receptors, ruling out synaptic interactions amongst MLIs. Thirdly, PSS is mimicked in whole-cell recording experiments by step depolarizations under current clamp. Activation of Ca-dependent K channels during the spike trains appears as a likely candidate to underlie PSS. Using immunocytochemistry, we find that one such channel type, KCa1.1, is present in the somato-dendritic and axonal compartments of MLIs. In cell-attached recordings, charybdotoxin and iberiotoxin significantly reduce the optically induced PSS, while TRAM-34 does not affect it, suggesting that KCa1.1 channels, but not KCa3.1 channels, contribute to PSS.
© 2022 Kassa et al.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

