Inherited human ITK deficiency impairs IFN-γ immunity and underlies tuberculosis
- PMID: 36326697
- PMCID: PMC9641312
- DOI: 10.1084/jem.20220484
Inherited human ITK deficiency impairs IFN-γ immunity and underlies tuberculosis
Abstract
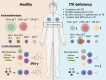
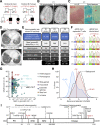
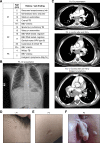
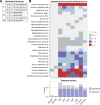
Inborn errors of IFN-γ immunity can underlie tuberculosis (TB). We report three patients from two kindreds without EBV viremia or disease but with severe TB and inherited complete ITK deficiency, a condition associated with severe EBV disease that renders immunological studies challenging. They have CD4+ αβ T lymphocytopenia with a concomitant expansion of CD4-CD8- double-negative (DN) αβ and Vδ2- γδ T lymphocytes, both displaying a unique CD38+CD45RA+T-bet+EOMES- phenotype. Itk-deficient mice recapitulated an expansion of the γδ T and DN αβ T lymphocyte populations in the thymus and spleen, respectively. Moreover, the patients' T lymphocytes secrete small amounts of IFN-γ in response to TCR crosslinking, mitogens, or forced synapse formation with autologous B lymphocytes. Finally, the patients' total lymphocytes secrete small amounts of IFN-γ, and CD4+, CD8+, DN αβ T, Vδ2+ γδ T, and MAIT cells display impaired IFN-γ production in response to BCG. Inherited ITK deficiency undermines the development and function of various IFN-γ-producing T cell subsets, thereby underlying TB.
© 2022 Ogishi et al.
Conflict of interest statement
Disclosures: Q. Philippot reported personal fees from Gilead outside the submitted work. S.J. Pelham reported other from Takeda UK Ltd outside the submitted work. No other disclosures were reported.
Figures

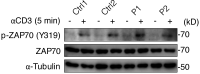


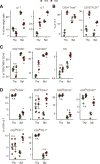

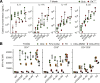



References
-
- Abel, L., Fellay J., Haas D.W., Schurr E., Srikrishna G., Urbanowski M., Chaturvedi N., Srinivasan S., Johnson D.H., and Bishai W.R.. 2018. Genetics of human susceptibility to active and latent tuberculosis: Present knowledge and future perspectives. Lancet Infect. Dis. 18:e64–e75. 10.1016/S1473-3099(17)30623-0 - DOI - PMC - PubMed
-
- Belkadi, A., Pedergnana V., Cobat A., Itan Y., Vincent Q.B., Abhyankar A., Shang L., El Baghdadi J., Bousfiha A., Exome/Array Consortium, et al. . 2016. Whole-exome sequencing to analyze population structure, parental inbreeding, and familial linkage. Proc. Natl. Acad. Sci. USA. 113:6713–6718. 10.1073/pnas.1606460113 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

