Zfp503/Nlz2 Is Required for RPE Differentiation and Optic Fissure Closure
- PMID: 36326727
- PMCID: PMC9645360
- DOI: 10.1167/iovs.63.12.5
Zfp503/Nlz2 Is Required for RPE Differentiation and Optic Fissure Closure
Abstract
Purpose: Uveal coloboma is a congenital eye malformation caused by failure of the optic fissure to close in early human development. Despite significant progress in identifying genes whose regulation is important for executing this closure, mutations are detected in a minority of cases using known gene panels, implying additional genetic complexity. We have previously shown knockdown of znf503 (the ortholog of mouse Zfp503) in zebrafish causes coloboma. Here we characterize Zfp503 knockout (KO) mice and evaluate transcriptomic profiling of mutant versus wild-type (WT) retinal pigment epithelium (RPE)/choroid.
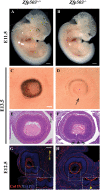
Methods: Zfp503 KO mice were generated by gene targeting using homologous recombination. Embryos were characterized grossly and histologically. Patterns and level of developmentally relevant proteins/genes were examined with immunostaining/in situ hybridization. The transcriptomic profile of E11.5 KO RPE/choroid was compared to that of WT.
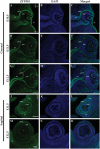
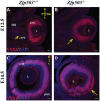
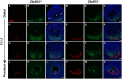
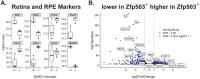
Results: Zfp503 is dynamically expressed in developing mouse eyes, and loss of its expression results in uveal coloboma. KO embryos exhibit altered mRNA levels and expression patterns of several key transcription factors involved in eye development, including Otx2, Mitf, Pax6, Pax2, Vax1, and Vax2, resulting in a failure to maintain the presumptive RPE, as evidenced by reduced melanin pigmentation and its differentiation into a neural retina-like lineage. Comparison of RNA sequencing data from WT and KO E11.5 embryos demonstrated reduced expression of melanin-related genes and significant overlap with genes known to be dynamically regulated at the optic fissure.
Conclusions: These results demonstrate a critical role of Zfp503 in maintaining RPE fate and optic fissure closure.
Conflict of interest statement
Disclosure:
Figures




References
-
- Patel A, Sowden JC.. Genes and pathways in optic fissure closure. Semin Cell Dev Biol. 2019; 91: 55–65. - PubMed
-
- Hero I. The optic fissure in the normal and microphthalmic mouse. Exp Eye Res. 1989; 49: 229–239. - PubMed
-
- Williamson KA, FitzPatrick DR.. The genetic architecture of microphthalmia, anophthalmia and coloboma. Eur J Med Genet. 2014; 57: 369–380. - PubMed
-
- Hsu P, Ma A, Wilson M, et al. .. CHARGE syndrome: a review. J Paediatr Child Health. 2014; 50: 504–511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

