Single-Molecule Trapping and Measurement in a Nanostructured Lipid Bilayer System
- PMID: 36326814
- PMCID: PMC9671048
- DOI: 10.1021/acs.langmuir.2c02203
Single-Molecule Trapping and Measurement in a Nanostructured Lipid Bilayer System
Abstract
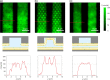
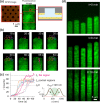
The repulsive electrostatic force between a biomolecule and a like-charged surface can be geometrically tailored to create spatial traps for charged molecules in solution. Using a parallel-plate system composed of silicon dioxide surfaces, we recently demonstrated single-molecule trapping and high precision molecular charge measurements in a nanostructured free energy landscape. Here we show that surfaces coated with charged lipid bilayers provide a system with tunable surface properties for molecular electrometry experiments. Working with molecular species whose effective charge and geometry are well-defined, we demonstrate the ability to quantitatively probe the electrical charge density of a supported lipid bilayer. Our findings indicate that the fraction of charged lipids in nanoslit lipid bilayers can be significantly different from that in the precursor lipid mixtures used to generate them. We also explore the temporal stability of bilayer properties in nanofluidic systems. Beyond their relevance in molecular measurement, such experimental systems offer the opportunity to examine lipid bilayer formation and wetting dynamics on nanostructured surfaces.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

